Abstract
Activation of brain α7 nicotinic acetylcholine receptors (α7 nAChRs) has broad therapeutic potential in CNS diseases related to cognitive dysfunction, including Alzheimer's disease and schizophrenia. In contrast to direct agonist activation, positive allosteric modulation of α7 nAChRs would deliver the clinically validated benefits of allosterism to these indications. We have generated a selective α7 nAChR-positive allosteric modulator (PAM) from a library of GABAA receptor PAMs. Compound 6 (N-(4-chlorophenyl)-α-[[(4-chloro-phenyl)amino]methylene]-3-methyl-5-isoxazoleacet-amide) evokes robust positive modulation of agonist-induced currents at α7 nAChRs, while preserving the rapid native characteristics of desensitization, and has little to no efficacy at other ligand-gated ion channels. In rodent models, it corrects sensory-gating deficits and improves working memory, effects consistent with cognitive enhancement. Compound 6 represents a chemotype for allosteric activation of α7 nAChRs, with therapeutic potential in CNS diseases with cognitive dysfunction.
Keywords: cognition, ion channels, memory, nicotine, schizophrenia
A range of neurological and psychiatric disorders involve cognitive deficit, including Alzheimer's disease, schizophrenia, and attention deficit hyperactivity disorder. Many studies support a significant role of α7 nicotinic acetylcholine receptors (α7 nAChRs) in these diseases, suggesting that modulation of α7 nAChRs represents an effective therapeutic strategy (1).
α7 nAChRs are rapidly desensitizing ligand-gated Ca2+ channels that are highly expressed in the hippocampus, a limbic structure intimately linked to cognition, attention processing, and memory formation (2). Deficits in α7 nAChR expression and function are associated with cognitive impairment in Alzheimer's disease and schizophrenia (3, 4) and with reduced cognitive performance in animal models (5). Consistent with these findings, activation of α7 nAChRs results in improved cognitive performance in both animal models and humans (6, 7), supporting the concept that identification of selective α7 nAChR activators may yield potential drugs for treating cognitive disorders (1).
Enhancement of α7 nAChR activity via positive allosteric modulation would provide the advantages of allosterism to treat patients afflicted by these therapeutically underserved CNS disorders. A key advantage of allosteric modulation is enhanced receptor activity only in the presence of the endogenous agonist, thereby preserving the temporal integrity of neurotransmission (8). Positive allosteric modulators (PAMs) have a long and successful clinical track record exemplified by the benzodiazepine family of drugs (e.g., diazepam), which function as allosteric modulators of GABAA receptors (9). Although several small-molecule α7 nAChR PAMs have been reported, including galantamine, 5-hydroxyindole, and PNU-120596 (10–12), none alone offers the characteristics of an ideal α7 nAChR PAM: selectivity, potency, and modulation accompanied by the maintenance of rapid receptor desensitization kinetics, a fundamental characteristic of these ligand-gated ion channels. For example, the Alzheimer's disease drug galantamine evokes very low efficacy positive modulation over a narrow concentration range in vitro and is nonselective (13, 14). Galantamine is also a nAChR channel blocker at slightly higher concentrations and has anticholinesterase activity, which is the original basis for its use in Alzheimer's disease (10). 5-hydroxyindole evokes more robust positive allosteric modulation of α7 nAChRs, but with potency in the millimolar range (11). PNU-120596, a recently reported selective α7 nAChR PAM, has behavioral activity in reversing sensory-gating deficits in rodents (12), helping to validate the concept that a selective α7 nAChR PAM may have efficacy in schizophrenia. However, positive modulation by PNU-120596 is accompanied by a profound retardation of the kinetics of desensitization, raising the potential for Ca2+-induced toxicity through excessive stimulation of α7 nAChRs (15, 16). PAMs with this property will likely have limited clinical usefulness.
Drug discovery efforts to identify novel α7 nAChR PAMs are hampered by technical difficulties associated with the rapid desensitization of native α7 nAChRs. To circumvent these problems, high-throughput screening methods have used mutated or chimeric α7 nAChRs in which channel kinetics are slowed to assist in detection of channel activity when using plate-reader assay formats (17, 18). By using this approach, which actually confounds the ability to detect compounds that preserve native desensitization kinetics, only PAMs like PNU-120596 and others that drastically alter channel kinetics have been identified to date (12, 19). Consequently, the discovery of selective α7 nAChR PAMs that closely preserve native channel kinetics remains an important but elusive goal in this field.
In addition to the difficulties of screening compounds for modulatory activity on α7 nAChRs, the identification of drug-like chemotypes on which selective α7 nAChR PAMs can be derived has been problematic. We had previously identified a series of GABAA receptor PAMs that evoke modulation at a site distinct from the benzodiazepine binding site (20), and had synthesized a small focused library of compounds in this structural series. Given the sequence homology between GABAA and nACh receptors (21), we hypothesized that compounds from our GABAA PAM library may also modulate α7 nAChRs. Applying this rationale, we screened this library for positive modulation of native human α7 nAChRs expressed in Xenopus oocytes by using standard two-electrode voltage-clamp electrophysiology. The advantage of this simple low-throughput method is that it can detect the native rapid kinetics of channel gating. By using this methodology, we identified a highly selective α7 nAChR PAM, compound 6 (N-(4-chlorophenyl)-α-[[(4-chloro-phenyl)amino]methylene]-3-methyl-5-isoxazoleacet-amide), from compounds that are PAMs of both GABAA and α7 nACh receptors. In contrast to PAMs like PNU-120596, currents enhanced by compound 6 retain the rapid kinetic and desensitization properties of the native channel. In addition, compound 6 does not induce toxicity in a cell line expressing α7 nAChRs, whereas PNU-120596 is highly toxic in this system. Consistent with its in vitro profile, compound 6 normalizes sensory-gating deficits, reverses MK-801-induced hyperlocomotion, and improves working memory in rodent models relevant to cognition and schizophrenia. Our translational design strategy of applying principles learned from creating novel PAMs of GABAA receptors has yielded a chemotype for the design of therapeutically useful α7 nAChR PAMs.
Results
Identification of a Selective α7 nAChR PAM.
We screened our library of novel GABAA receptor PAMs (20) to identify compounds that modulated submaximal nicotine-evoked currents at human α7 nAChRs by using standard Xenopus oocyte electrophysiology. A screening hit, compound 1, evoked concentration-dependent high efficacy positive modulation of both α7 nACh and GABAA receptors, but did not modulate α4β2 nAChRs or 5-hydroxytryptamine 3A (5-HT3A) receptors [supporting information (SI) Fig. 6].
Compound 1 provided the initial template for structure–activity relationship studies with the initial goals (i) to increase selectivity vs. GABAA receptors to avoid potential side effects (e.g., sedation) associated with robust GABAA receptor stimulation, and (ii) to generate a compound with adequate bioavailability for behavioral studies (SI Fig. 7 and SI Materials and Methods). Three groups around the core enaminone of compound 1 were chemically modified: the aryl ketone, the ethyl ester, and the aniline to improve selectivity for the α7 nAChR and enhance bioavailability. Compound 6 resulted from these chemical modifications and was chosen for further characterization.
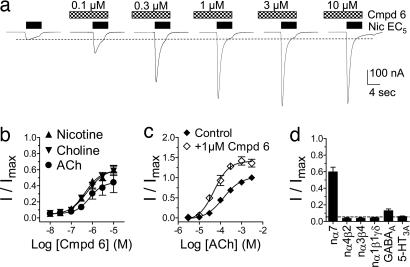
Compound 6 (0.1 nM to 10 μM) did not displace the binding of the selective α7 nAChR antagonist 125I-labeled α-bungarotoxin to rat brain tissue as quantified autoradiographically (SI Fig. 8 and SI Materials and Methods), nor did it directly evoke currents in oocytes expressing human α7 nAChRs (Fig. 1a). These data suggest that compound 6 does not bind to or activate α7 nAChRs via the orthosteric site. However, compound 6 evoked concentration-dependent positive modulation of agonist-evoked currents in oocytes expressing human α7 nAChRs. In one series of experiments (e.g., Fig. 1a), the concentration of the agonist was fixed at 5% (EC5) of the maximal current (Imax) and the effects of compound 6 were assessed. Compound 6 evoked positive modulation of EC5 currents induced by acetylcholine (ACh), choline, and nicotine, with EC50 (and nH) values for positive modulation of 0.7 ± 0.2 μM (1.4 ± 0.9), 0.6 ± 0.1 μM (1.2 ± 0.3), and 0.5 ± 0.1 μM (1.2 ± 0.3), respectively. Maximal extent of efficacy of positive modulation of EC5-evoked control currents peaked at 45%, 60%, and 60% of the maximal agonist response for these three agonists, respectively (Fig. 1 a and b). In a second series of experiments, a fixed concentration of compound 6 (1 μM) induced a leftward shift in the concentration–response curve for ACh, resulting in an ≈2.7-fold reduction in the EC50 values for ACh from 136 ± 0.1 μM (control) to 50 ± 0.1 μM (in the presence of compound 6) and a slight increase in nH values from 1.0 ± 0.2 to 1.2 ± 0.3, respectively (Fig. 1c). Positive modulation evoked by compound 6 at α7 nAChRs in oocytes was independent of membrane voltage and did not change the reversal potential, suggesting that compound 6 does not alter ion selectivity (SI Fig. 9).
Fig. 1.
Compound 6 selectively enhances wild-type human α7 nAChR-mediated currents in Xenopus oocytes. (a) Typical recording illustrating positive modulation of submaximal (EC5) nicotine-evoked currents by compound 6 at human α7 nAChRs. Pretreatments with compound 6 (30 sec) were followed by coapplication with nicotine (5 sec). Nic, nicotine. (b) Concentration–response relationships for compound 6-evoked modulation of ACh, choline, and nicotine (EC5) elicited currents at human α7 nAChRs. (c) ACh concentration–response curves in the presence or absence of compound 6 (Cmpd 6). (d) Comparison of maximal modulation evoked by compound 6 (10 μM) at α7, α4β2, α3β4, and α1β1γδ nACh; GABAA α1β2γ2L and 5-HT3A receptors. All data in each experiment are from four oocytes.
Compound 6 did not evoke modulation of EC5-evoked currents at human α4β2, rat α3β4, and mouse α1β1γδ nAChR subtypes or human 5-HT3A receptors expressed in oocytes (Fig. 1d). In contrast, compound 6 evoked low efficacy concentration-dependent positive modulation of submaximal GABA-evoked currents at human GABAA α1β2γ2L receptors expressed in oocytes. Maximal efficacy for modulation of EC5-evoked GABA currents by compound 6 peaked at ≈15% of the maximal GABA response with an EC50 value of 6.3 ± 0.2 μM (SI Fig. 10). These data suggest that compound 6 is selective for human α7 nAChRs among the ligand-gated ion channels tested.
Compound 6 also evoked positive modulation of choline-evoked currents in SH-SY5Y cells transfected with α7 nAChRs (“SH-α7 cells”) (22), with a 1.8-fold increase over control (SI Fig. 11 and SI Materials and Methods). These modulated currents were blocked by 10 nM of the α7 nAChR antagonist methyllycaconitine, suggesting that these effects were mediated by α7 nAChRs.
Characterization of Macroscopic Kinetics of Desensitization.
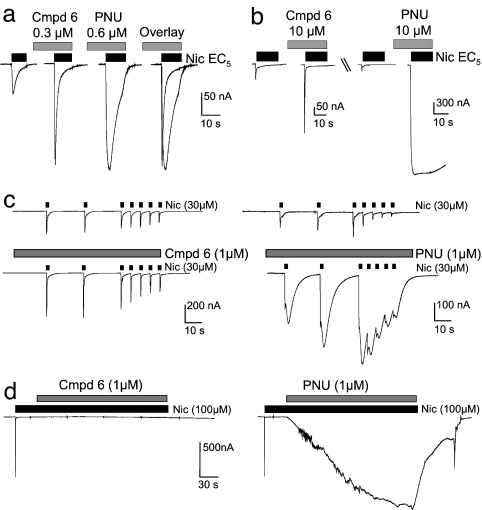
At α7 nAChRs in oocytes, compound 6 potentiated agonist-evoked currents while preserving the native channel kinetics, including rapid activation and rapid desensitization (Fig. 2 a and b). In contrast, currents modulated by the reference PAM, PNU-120596, were accompanied by a significant retardation in channel kinetics (Fig. 2 a and b), as previously reported (12). To further test the properties of modulation, we simulated temporal synaptic events by repeated brief agonist exposures (0.5 and 0.02 Hz). Compound 6 increased currents relative to controls at both frequencies during coapplication of nicotine, without changing the macroscopic kinetics (Fig. 2c). In contrast, PNU-120596 increased currents with a profound slowing of desensitization kinetics; currents did not return to baseline during this temporal sequence. Finally, we tested compound 6 for the potential to reverse desensitization evoked by extended exposure to the agonist, as reported for PNU-120596 (12). Unlike PNU-120596, compound 6 did not reverse desensitization evoked by a 2.5-min exposure to nicotine (Fig. 2d).
Fig. 2.
Maintenance of rapid native kinetics and desensitization after modulation by compound 6 at human α7 nAChRs expressed in Xenopus oocytes. Experiments were performed on four oocytes. (a and b) Comparison of macroscopic kinetics after positive modulation of (EC5) nicotine-evoked currents by compound 6 and PNU-120596 at concentrations displaying similar extent of modulation (a) and 10 μM (b). (c) Effect of compound 6 (Left) and PNU-120596 (Right) on a timed sequence of brief (1-sec duration) exposures to nicotine. (d) Effect of compound 6 (Left) and PNU-120596 (Right) exposures (2.5-min duration) after desensitization by a prolonged exposure to nicotine (30 sec). Cmpd 6, compound 6; PNU, PNU-120596; Nic, nicotine.
Cytotoxicity of Selective α7 nAChR PAMs.
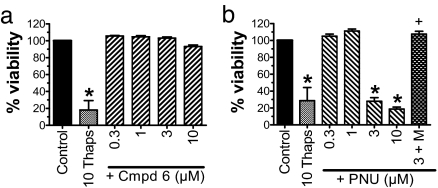
Compound 6 and PNU-120596 were assayed for potential cytotoxicity by using the SH-α7 cell line (22). Before the toxicity assays, we tested these cells by using patch-clamp electrophysiology to confirm (i) the presence of functional α7 nAChRs, and (ii) the functional positive modulation of agonist-evoked currents by compound 6 and PNU-120596, as described above (SI Fig. 11 and SI Materials and Methods). In the toxicity assays, SH-α7 cultures were exposed to compound 6 or PNU-120596 for 24 h at a dose range of 0.3 to 10 μM, the range previously shown to evoke positive receptor modulation. Exposure to compound 6 (≤10 μM) did not reduce SH-α7 viability, whereas exposure to PNU-120596 (3 and 10 μM) induced a significant reduction in viability, similar in extent to that evoked by thapsigargin (Fig. 3) (23). The cytotoxicity produced by PNU-120596 (3 μM) was blocked by 10 nM methyllycaconitine, suggesting an α7 nAChR-mediated mechanism. Methyllycaconitine alone had no effect on cell viability (data not shown). Although we did not add a nicotinic agonist to our assays, the growth media contained ≈0.1 mM choline, an α7 nAChR-selective agonist (24). As confirmed in our patch-clamp data (SI Fig. 11 and SI Materials and Methods), choline in the presence of PNU-120596 will evoke robust prolongation of channel opening in these cells (12).
Fig. 3.
Compound 6 does not change SH-SY5Y-α7 cell viability. SH-SY5Y-α7 cells were exposed to compound 6 (Cmpd 6) (a), PNU-120596 (PNU) (b), and the positive control, 10 μM thapsigargin (10 Thaps), for 24 h. Data represent mean ± SEM; n = 4 per group. ∗, P < 0.01 compared with control; †, P < 0.01 compared with PNU-120596 (3 μM), one-way ANOVA, Bonferroni's post hoc test. M, 10μM methylcaconitine.
In Vivo Characterization of Compound 6.
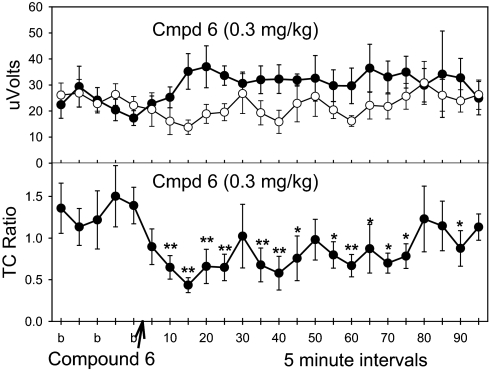
We assayed compound 6 in three rodent models relevant to cognition and schizophrenia. In the DBA/2 mouse model of sensory-gating deficit, compound 6 at 0.3 and 1 mg/kg, i.v., significantly reduced the ratio of test amplitude to conditioning amplitude (TC ratio) up to 90 min after administration, reflecting a dose-dependent normalization of sensory gating (Fig. 4 and SI Fig. 12 a and b). Under similar conditions, PNU-120596 was active in the DBA/2 model at 1 mg/kg but not 0.3 mg/kg and had a shorter duration of action than compound 6 (SI Fig. 13). The reduction in TC ratio (specifically, the test amplitude component) evoked by compound 6 was fully blocked by α-bungarotoxin (1.25 nM, intracerebroventricularly) (SI Fig. 14).
Fig. 4.
Normalization of sensory-gating deficits in DBA/2 mice by compound 6. Data are presented as evoked responses (Upper) of conditioning amplitude (closed circles), test amplitude (open circles), and TC ratio (Lower). Statistically significant normalization of sensory gating by compound 6 (Cmpd 6) at 0.3 mg/kg, i.v., was observed in the TC ratio. Data are the mean ± SEM; n = 8 per group. ∗, P < 0.05; ∗∗, P < 0.01, Fisher's least significant difference.
In the MK-801-induced hyperlocomotion model in NSA mice, compound 6 at 0.3 to 3 mg/kg orally significantly reversed the effect of MK-801 (SI Fig. 15a). The positive control haloperidol was also active but only at a dose that reduced locomotor activity when administered alone. Compound 6 at 0.1 to 10 mg/kg orally alone did not change locomotor activity (SI Fig. 15b).
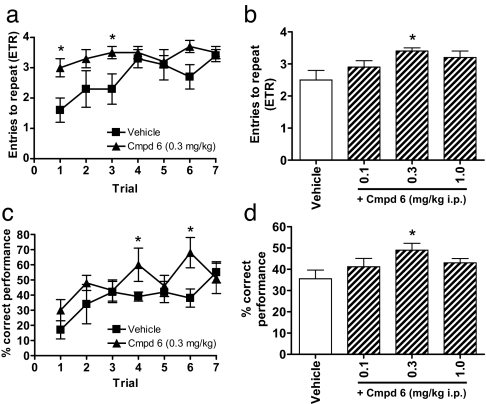
The eight-arm radial maze is a widely used animal model for testing hippocampal-based cognitive performance. In the memory acquisition paradigm, rats treated with compound 6 at 0.3 mg/kg, i.p., showed significant improvement in performance over vehicle-treated controls observed in both choice accuracy (Fig. 5 a and b) and percent correct performance (Fig. 5 c and d) on individual and averaged block trials. No significant differences were observed for any other measure.
Fig. 5.
Compound 6 improves memory acquisition of rats in the eight-arm radial maze. Compound 6 (Cmpd 6) at 0.3 mg/kg, i.p., significantly increases the entries to repeat (ETR) (a and b) and percent correct performance (c and d) measures compared with vehicle controls for both individual (Left) and averaged (first six trials) (Right) trial blocks. Data are mean ± SEM; n = 6–7 per group. ∗, P < 0.05 compared with vehicle control, one-way ANOVA, Dunnett's post hoc test.
In DBA/2 mice, brain penetration of compound 6 was determined at the behaviorally active dose of 0.3 mg/kg, i.v. Brain concentrations ranged from ≈1 μM at 10 min to ≈0.3 μM at 90 min after injection, corresponding to the time interval in which the TC ratios were measured (SI Fig. 16). In NSA mice, brain penetration of compound 6 was determined at the behaviorally active dose of 0.3 mg/kg orally. Brain concentrations ranged from a peak (Cmax) of ≈0.3 to ≈0.15 μM during the 120 to 150 min after administration interval that corresponded to the 30-min span during which hyperlocomotion was monitored (SI Fig. 16).
Discussion
Compound 6 represents a drug-like α7 nAChR PAM that robustly potentiates channel currents while preserving the rapid kinetic and desensitization properties of the channel. It is orally bioavailable and is active in rodent models relevant to both schizophrenia and cognition (25, 26).
Our rationale to identify an α7 nAChR PAM began with the hypothesis that the GABAA and α7 nACh receptors exhibit sufficient homology to allow compounds that modulate both receptors. This concept is supported by the existence of PAMs with nonselective modulation across the ligand-gated ion channel family (11, 27). The initial screening hit compound 1 modulated both GABAA and α7 nACh receptors, consistent with a conserved binding pocket. The increased selectivity for α7 nAChRs observed with compound 6 suggests that either compound 6 has moved away from the conserved binding pocket or the mechanism of modulation has changed. Currently, our experiments do not address the potential that compound 6 may share a binding site with other reported PAMs. Future studies using mutant α7 nAChRs or specific radioligands may provide more information regarding the mechanism of action on channel kinetics.
A key characteristic of native α7 nAChR activity is rapid channel kinetics and desensitization. An ideal positive modulator will increase currents without altering these native kinetics. A PAM, such as compound 6, which enhances α7 nAChR-mediated current amplitude while retaining rapid channel kinetics, will maintain the temporal and kinetic patterns of native synaptic signaling (8). Identification of novel PAMs that preserve native kinetics represents a significant technical hurdle for high-throughput screening methods, requiring a combination of rapid agonist application and subsecond detection of channel activity. Assay formats using “slowed” receptors (17, 18) may not detect compounds such as compound 6. We instead used native receptors with a traditional electrophysiology format, incorporating a customized fast drug application system (28), allowing high-resolution detection of PAMs that retain rapid kinetics.
Maintenance of rapid channel kinetics is also particularly important because α7 nAChRs are highly permeable to Ca2+, an ion associated with cell toxicity (16). Ca2+-mediated toxicity related to α7 nAChRs with slowed kinetics has been previously reported in the form of the receptor mutations L250T and/or V251T, which evoke cytotoxicity in SH-SY5Y cells and lethality in mutant mice because of Ca2+-mediated apoptosis (15, 29). The L250T mutation results in a constitutive prolonged opening of α7 nAChR channels (30), similar to the prolonged opening evoked by PNU-120596 in the presence of an agonist, predicting that PNU-120596 may have similar toxic effects in the presence of an agonist. Our data show that PNU-120596 is cytotoxic to the SH-α7 cell line, consistent with this general hypothesis. Although we did not add a nominal nicotinic agonist to our assay, the growth media contained ≈0.1 mM choline, an α7 agonist that would evoke prolonged channel opening in the presence of PNU-120596 (24). In contrast, compound 6 is not cytotoxic to SH-α7 cells over the same range of concentrations tested. This suggests that PAMs that preserve fast kinetics, like compound 6, may selectively modulate α7 nAChRs to enhance synaptic neurotransmission while minimizing the risk of Ca2+-related toxicity. Nevertheless, definitive in vivo toxicity studies must be done to confirm this possibility before the therapeutic potential of PAMs such as compound 6 can be assessed.
The DBA/2 mouse model of sensory-gating deficit is highly correlated to a similar deficit in schizophrenia (31). Unlike several rodent-gating models that use pharmacological manipulation to elicit sensory deficits, DBA/2 mice have a reduction in α7 nAChR expression and intrinsic sensory-gating deficits, properties also observed in schizophrenics (3, 32). Consistent with the role of α7 nAChRs in sensory gating, compound 6 significantly reverses sensory-gating deficits in DBA/2 mice. This improvement in TC ratio is produced primarily by a decrease in test amplitude, which is consistent with previous studies with GTS-21, the α7 nAChR partial agonist, which also induces a similar effect (31). Consistent with an α7 nAChR-mediated effect, α-bungarotoxin blocked the improvements induced by compound 6 in sensory gating.
The antagonism of NMDA receptors by phencyclidine and its analog, MK-801, has been shown to induce schizophrenic-like symptoms in humans and hyperlocomotion in rodents (33, 34). Although the exact mechanism for MK-801-induced hyperlocomotion in rodents remains obscure, the assay may have heuristic value in that clinically used neuroleptics, including haloperidol, are active in this model (33). Compound 6 significantly reduces MK-801-induced hyperlocomotion, suggesting potential therapeutic usefulness in alleviating schizophrenic symptoms related to NMDA hypofunction. The dose–response relationship for compound 6 revealed an optimally effective dose (e.g., reduced activity at higher doses resulting in a “bell-shaped” dose–response relationship) in this model, a phenomenon observed in other paradigms evaluating nicotinic agonists and theorized to be related to an optimal degree of nicotinic receptor stimulation (35, 36).
The rat eight-arm radial maze has been used extensively to study the mechanisms involved in hippocampal-based working memory (26). Compound 6 shows significant improvement in both choice accuracy and percent correct performance in this model, with an optimal dose range for improvement in performance, as noted above. These results are consistent with the hypothesis that α7 nAChRs are involved in cognitive function, perhaps by modulating attention and that activating these receptors may help correct cognitive deficits (26).
Pharmacokinetic studies suggest that compound 6 reaches brain concentrations sufficient to modulate α7 nAChRs in these behavioral assays, and that these brain levels correspond to the time during which significant behavioral effects were observed. For example, in the sensory-gating studies, compound 6 declines to 0.3 μM in the brain 90 min after i.v. administration of 0.3 mg/kg of compound 6. This concentration (0.3 μM) correlates with positive modulation at α7 nAChRs measured in vitro from 5% (control) to ≈20% of the Imax. Importantly, modulation of GABAA receptors by 0.3 μM compound 6 is not significantly different from control, suggesting that low efficacy modulation at α7 nAChRs, and not GABAA receptors, is sufficient to correct the sensory-gating deficit (SI Fig. 10). Additional support for the hypothesis that GABAA receptor-mediated activity is minimal or nonexistent is the observation that compound 6 does not induce rotarod failures at brain levels of up to ≈17 μM (data not shown). More importantly, these results imply that a selective PAM that does not perturb the rapid native channel kinetics of α7 nAChRs, such as compound 6, has sufficient efficacy to restore sensory gating despite an ≈50% reduction of hippocampal α7 nAChRs in DBA/2 mice (32) and that potentiation of ambient concentrations of endogenous agonist is sufficient to restore function in this model.
The therapeutic usefulness of targeting α7 nAChRs for cognitive disorders is further supported by evidence suggesting α7 nAChRs mediate the attention deficit underlying impaired cognition observed in α7 nAChR knockout mice (37). Recent clinical trials have demonstrated that GTS-21 significantly improves cognition in schizophrenics, supporting the proposal that stimulation of α7 nAChRs will have beneficial effects in both sensory inhibition and cognition (38). Correction of cognitive deficits, thought to be a major determinant and predictor of work disability and poor community adaptation in schizophrenia, could be a major breakthrough in aiding the return of these patients into mainstream society (39). Currently, there are no drugs that adequately or specifically address the cognitive deficits of schizophrenia.
We used a translational strategy to generate compound 6, a selective α7 nAChR PAM that preserves the rapid native kinetic properties of channel activity and avoids potential Ca2+-mediated neurotoxicity. Our data suggest that therapeutic agents with the properties of compound 6 may have usefulness against CNS disorders involving cognitive deficits such as schizophrenia. Future studies may also show potential usefulness in other neuropathological disorders related to α7 nAChRs, including Alzheimer's disease, attention deficit hyperactivity disorder, and other diseases involving cognitive dysfunction.
Materials and Methods
Oocyte Electrophysiology.
cDNA clones were provided as gifts: α7, α4, and β2 nAChRs from Jon Lindstrom (University of Pennsylvania, Philadelphia, PA); GABAA receptor subunits from CoCensys, Inc. (Irvine, CA); and α3, β4, α1, β1, γ, and δ nAChRs from James Boulter (University of California, Los Angeles, CA). 5-HT3A was purchased from the American Type Culture Collection (Manassas, VA). mRNA transcription was performed with the mMessage mMachine system (Ambion, Austin, TX) and diluted to ≈1 μg/μl.
Oocytes were obtained from Xenopus laevis frogs by using procedures approved and monitored by the University of California, Irvine Institutional Animal Care and Use Committee. Preparation, microinjection, and maintenance of oocytes were as described previously (40). Individual oocytes were injected with 0.005 to 50 ng of each subunit mRNA as follows (ratio of subunits in parentheses): α7 nAChR; α4β2 nAChRs (1:1); α3β4 nAChRs (1:1); α1β1γδ nAChRs (1:1:1:1); GABAA receptor subunit combinations (α2β3γ2L and α1β2γ2L) (5:1:1); and 5-HT3A receptors. Two-electrode voltage-clamp recordings were made 3 to 14 days after mRNA injections at a holding voltage of −70 mV unless specified. The nACh and 5-HT3A receptor recordings were performed in Ca2+-free Ringer's solution (115 mM NaCl/2mM KCl/1.8mM BaCl2/5 mM Hepes, pH 7.4) to limit Ca2+-activated chloride currents. Atropine (0.5 μM) was used in recordings by using ACh to prevent endogenous muscarinic currents. The recordings using GABA were performed in standard Ringer's solution (115 mM NaCl/2 mM KCl/1.8 mM CaCl2/5 mM Hepes, pH 7.4). In all oocyte recordings, drug and wash solutions were applied by using a custom-designed microcapillary “linear array,” which allows rapid (subsecond) application of agonists (28). Currents were recorded on a chart recorder and/or PC-based computer (PClamp 9.0; Molecular Devices, Sunnyvale, CA). Concentration response curves were reported as I/Imax, where I is the amount of current given by a selected concentration of drug and Imax is the amount of maximal current. Concentration–effect data were fit to a four-parameter logistic equation (GraphPad Software, San Diego, CA).
Cell Viability.
SH-SY5Y-α7 cells (passage 6) were a gift from Christian Fuhrer (University of Zurich, Zurich, Switzerland). The cells were maintained in “complete growth medium” as described (SI Materials and Methods). “Experimental medium” consisted of complete growth medium with 0.5% FBS.
SH-SY5Y-α7 cells were plated on 96-well plates at a density of 15,000 cells per well (100 μl of 1.5 × 105 cells per ml) in complete growth medium and placed into a 37°C incubator for 20 to 24 h. Complete growth medium then was replaced with experimental medium alone (“compound free”) or containing appropriate concentrations of compound and returned to the 37°C incubator for 20 to 24 h. The medium was then replaced with fresh experimental medium and 20 μl per well MTS solution (CellTiter96 cell viability kit; Promega, Madison, WI) and returned to the 37°C incubator for 3 h, after which the plate was read on a microplate spectrophotometer at an absorbance of 490 nm. For all data analysis, data were normalized to untreated compound-free wells (100% cell viability) and a background absorbance taken from wells containing experimental medium and MTS solution.
Auditory Evoked Potential Model of Sensory Gating.
Male DBA/2 mice (20–25 g; Harlan, San Diego, CA) were prepared as described (31). Three parameters were measured: conditioning amplitude, the magnitude of the response to the first, or conditioning stimulus of paired identical stimuli delivered 0.5 sec apart; test amplitude, the magnitude of the response to the second, or test stimulus; and TC ratio, the ratio of the test amplitude to conditioning amplitude. Formulation of compound 6 at 0.1 and 0.3 mg/kg was in 0.2% DMSO/0.8% Solutol/99% saline and at 1.0 mg/kg was in 1% DMSO/1% Solutol/98% saline. PNU-120596 (0.3 and 1.0 mg/kg) was formulated in 5% DMSO/5% Solutol/90% normal saline. Five baseline “b” records were taken before compound 6 or PNU-120596 administration. Compound 6 or PNU-120596 were administered at 4 ml/kg, i.v., at various doses. α-Bungarotoxin (1.25 nM) was administered by intracerebroventricular injection in 1 μl of saline before injection of compound 6 (0.3 mg/kg, i.v.).
Eight-Arm Radial Maze Task.
Adult male Sprague–Dawley rats (250–300 g; Charles River, Wilmington, MA) were housed four per cage and were food-restricted to maintain their body weights at ≈90% of free-feeding levels, adjusted for growth. This protocol was approved by the University of California, Irvine, Institutional Animal Care and Use Committee. Testing used an automated radial maze (Coulbourn Instruments, Allentown, PA) consisting of a 30-cm central octagon platform with eight radial arms (10 × 38 cm) bordered by Plexiglas in a room with fixed extramaze visual cues. Ends of the arms were baited with a 45-mg sugar pellet. Testing for memory acquisition used a partial baiting paradigm (four of eight arms randomly selected and baited) for a total of seven sessions. Arm entries were recorded if at least two of the rat's paws and half a body crossed the threshold of the arm, and (correct) arm completion was also noted whether the sugar pellet was eaten. The training trial was considered complete when all four pellets were consumed or 5 min had passed. Rats were divided into four groups that were injected daily (i.p.), starting with the first day of shaping, with either vehicle or compound 6 (0.1, 0.3, or 1.0 mg/kg) 30 min before testing. The timing of test drug injections was chosen to allow peak brain levels to be present during the time when the behavioral test was performed. Scoring included working memory errors and reference memory errors (26). Percent correct performance was calculated by using the following formula: % correct performance = no. of correct choices/(maximum no. of correct choices + total no. of errors) × 100 (41). Choice accuracy was also measured by using “entries to repeat,” which was the number of correct baited arm entries until a working memory error was committed, with “4” considered a perfect working memory score. Response latency (seconds per entry) and trial duration (seconds) were also recorded.
SI.
Additional data can be found in SI Figs. 6–16 and SI Materials and Methods.
Acknowledgments
We thank Dr. Jose Aguilar, Dr. David Putman, Dr. Olivier Dasse, Wen Li, and Jin Huang for technical assistance and Drs. Jon Lindstrom, Jim Boulter, and Christian Fuhrer for generous gifts. This work was supported by University of California Discovery Grant bio04-10469 (to K.W.G.).
Abbreviations
- α7 nAChR
α7 nicotinic acetylcholine receptor
- PAM
positive allosteric modulator
- 5-HT3A
5-hydroxytryptamine 3A
- ACh
acetylcholine
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt
- TC ratio
ratio of test amplitude to conditioning amplitude.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701321104/DC1.
References
- 1.Mazurov A, Hauser T, Miller CH. Curr Med Chem. 2006;13:1567–1584. doi: 10.2174/092986706777442011. [DOI] [PubMed] [Google Scholar]
- 2.Gotti C, Fornasari D, Clementi F. Prog Neurobiol. 1997;53:199–237. doi: 10.1016/s0301-0082(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 3.Freedman R, Hall M, Adler LE, Leonard S. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- 4.Guan ZZ, Zhang X, Ravid R, Nordberg A. J Neurochem. 2000;74:237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- 5.Bettany JH, Levin ED. Pharmacol Biochem Behav. 2001;70:467–474. doi: 10.1016/s0091-3057(01)00643-8. [DOI] [PubMed] [Google Scholar]
- 6.Levin ED, Bettegowda C, Blosser J, Gordon J. Behav Pharmacol. 1999;10:675–680. doi: 10.1097/00008877-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, Clody DE, Burnett AL. Neuropsychopharmacology. 2003;28:542–551. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 8.Christopoulos A. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 9.Mohler H, Fritschy JM, Rudolph U. J Pharmacol Exp Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 10.Maelicke A. Dement Geriatr Cogn Disord. 2000;11(Suppl 1):11–18. doi: 10.1159/000051227. [DOI] [PubMed] [Google Scholar]
- 11.Zwart R, De Filippi G, Broad LM, McPhie GI, Pearson KH, Baldwinson T, Sher E. Neuropharmacology. 2002;43:374–384. doi: 10.1016/s0028-3908(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 12.Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann WE, Piotrowski DW, et al. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samochocki M, Hoffle A, Fehrenbacher A, Jostock R, Ludwig J, Christner C, Radina M, Zerlin M, Ullmer C, Pereira EF, et al. J Pharmacol Exp Ther. 2003;305:1024–1036. doi: 10.1124/jpet.102.045773. [DOI] [PubMed] [Google Scholar]
- 14.Texido L, Ros E, Martin-Satue M, Lopez S, Aleu J, Marsal J, Solsona C. Br J Pharmacol. 2005;145:672–678. doi: 10.1038/sj.bjp.0706221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukas RJ, Lucero L, Buisson B, Galzi JL, Puchacz E, Fryer JD, Changeux JP, Bertrand D. Eur J Neurosci. 2001;13:1849–1860. doi: 10.1046/j.0953-816x.2001.01560.x. [DOI] [PubMed] [Google Scholar]
- 16.Orrenius S, Zhivotovsky B, Nicotera P. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 17.Placzek AN, Grassi F, Meyer EM, Papke RL. Mol Pharmacol. 2005;68:1863–1876. doi: 10.1124/mol.105.016402. [DOI] [PubMed] [Google Scholar]
- 18.Craig PJ, Bose S, Zwart R, Beattie RE, Folly EA, Johnson LR, Bell E, Evans NM, Benedetti G, Pearson KH, et al. Eur J Pharmacol. 2004;502:31–40. doi: 10.1016/j.ejphar.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Sher E, Zwart R, De Filippi G, Baldwinson T, Pearson K, McPhie G, Keenan M, O'Neill M, Fucile S, Broad L. Soc Neurosci Abstr. 2005;31:951.10. [Google Scholar]
- 20.Johnstone TB, Hogenkamp DJ, Coyne L, Su J, Halliwell RF, Tran MB, Yoshimura RF, Li WY, Wang J, Gee KW. Nat Med. 2004;10:31–32. doi: 10.1038/nm967. [DOI] [PubMed] [Google Scholar]
- 21.Connolly CN, Wafford KA. Biochem Soc Trans. 2004;32:529–534. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- 22.Charpantier E, Wiesner A, Huh KH, Ogier R, Hoda JC, Allaman G, Raggenbass M, Feuerbach D, Bertrand D, Fuhrer C. J Neurosci. 2005;25:9836–9849. doi: 10.1523/JNEUROSCI.3497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arai K, Lee SR, van Leyen K, Kurose H, Lo EH. J Neurochem. 2004;89:232–239. doi: 10.1111/j.1471-4159.2004.02317.x. [DOI] [PubMed] [Google Scholar]
- 24.Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin LF, Kem WR, Freedman R. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 26.Levin ED, McClernon FJ, Rezvani AH. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 27.Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- 28.Hawkinson JE, Drewe JA, Kimbrough CL, Chen JS, Hogenkamp DJ, Lan NC, Gee KW, Shen KZ, Whittemore ER, Woodward RM. Mol Pharmacol. 1996;49:897–906. [PubMed] [Google Scholar]
- 29.Orr-Urtreger A, Broide RS, Kasten MR, Dang H, Dani JA, Beaudet AL, Patrick JW. J Neurochem. 2000;74:2154–2166. doi: 10.1046/j.1471-4159.2000.0742154.x. [DOI] [PubMed] [Google Scholar]
- 30.Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, Bertrand S, Ballivet M, Changeux JP. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 31.Stevens KE, Kem WR, Mahnir VM, Freedman R. Psychopharmacology. 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 32.Stevens KE, Freedman R, Collins AC, Hall M, Leonard S, Marks MJ, Rose GM. Neuropsychopharmacology. 1996;15:152–162. doi: 10.1016/0893-133X(95)00178-G. [DOI] [PubMed] [Google Scholar]
- 33.Adriani W, Felici A, Sargolini F, Roullet P, Usiello A, Oliverio A, Mele A. Exp Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- 34.Morris BJ, Cochran SM, Pratt JA. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Picciotto MR. Trends Pharmacol Sci. 2003;24:493–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- 36.Newhouse PA, Potter A, Singh A. Curr Opin Pharmacol. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Eur Neuropsychopharmacol. 2007;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, et al. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 39.Bowie CR, Harvey PD. Curr Opin Investig Drugs. 2006;7:608–613. [PubMed] [Google Scholar]
- 40.Whittemore ER, Yang W, Drewe JA, Woodward RM. Mol Pharmacol. 1996;50:1364–1375. [PubMed] [Google Scholar]
- 41.Ortega-Alvaro A, Gibert-Rahola J, Mico JA. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:104–111. doi: 10.1016/j.pnpbp.2005.08.020. [DOI] [PubMed] [Google Scholar]