Abstract
Insulin-like growth factors (IGFs) I and II are important regulators of cell proliferation and differentiation. After birth, plasma IGFs, representing mostly liver-derived IGFs, circulate in ternary complexes of 150 kDa consisting of one molecule each of IGF, IGF-binding protein (IGFBP) 3, and an acid labile subunit (ALS). Onset of ALS synthesis after birth is the primary factor driving the formation of ternary complexes. Capture of IGFs by ALS is thought to allow the development of a plasma reservoir without negative effects such as hypoglycemia and cell proliferation. To evaluate the importance of ALS and ternary complexes, we have created mice in which the ALS gene has been inactivated. The mutation was inherited in a Mendelian manner, without any effects on survival rates and birth weights. A growth deficit was observed in null mice after 3 weeks of life and reached 13% by 10 weeks. This modest phenotype was observed despite reductions of 62 and 88% in the concentrations of plasma IGF-I and IGFBP-3, respectively. Increased turnover accounted for these reductions because indices of synthesis in liver and kidney were not decreased. Surprisingly, absence of ALS did not affect glucose and insulin homeostasis. Therefore, ALS is required for postnatal accumulation of IGF-I and IGFBP-3 but, consistent with findings supporting a predominant role for locally produced IGF-I, is not critical for growth. This model should be useful to determine whether presence of ALS is needed for other actions of liver-derived IGF-I and for maintenance of homeostasis in presence of high circulating levels of IGF-II.
Insulin-like growth factors (IGFs) I and -II regulate cellular processes such as proliferation, apoptosis, and differentiation (1, 2). In early fetal life, these actions are predominantly carried out by locally produced IGFs. Later in life, plasma IGFs, most of which are synthesized by liver, are thought to supplement local production and to convey some of the effects of factors such as growth hormone (GH) and nutrition. These IGFs inputs are determined by vascular supply and permeability of tissue, by the concentration of IGFs, and, perhaps most importantly, by their molecular form. The latter is affected dramatically by development. Before birth, IGFs form binary complexes of 40–50 kDa with members of a family of IGF-binding proteins (IGFBPs) (2–5). After birth, however, synthesis of the acid labile subunit (ALS) by liver sequesters most IGFs into ternary complexes of 150 kDa consisting of one molecule each of IGF, IGFBP-3, or IGFBP-5, and ALS (4–7). Binary complexes can traverse capillary endothelia to deliver bioactive IGFs to tissues whereas ternary complexes are confined to the vasculature and have extended half-lives (2–4). These changes in molecular form are thought to allow IGFs to accumulate in postnatal plasma to levels reaching 1,000-fold that of insulin without nonspecific effects such as hypoglycemia (4, 8).
Ternary complexes are almost completely absent in plasma of GH-deficient animals (9). This is because plasma ALS and IGF-I originate mostly from liver, where transcription of both genes is GH-dependent (10, 11). GH, however, regulates directly or indirectly many other genes and processes (12), and, therefore, abnormalities of GH-deficient animals cannot be attributed only to low levels of ALS, IGF-I, and ternary complexes. Recent findings raising the possibility that ALS may have roles other than stabilization of plasma IGFs include detection of ALS mRNA in cartilaginous and membranous bone, and in kidney of fetal rats (13), and presence of significant levels of ALS in wound filtrates of adult rats, and in human synovial and ovarian fluids (14, 15).
To study the role of ALS and the importance of having IGFs in ternary complexes, we used targeted gene inactivation to create a strain of mice harboring a null mutation of the ALS gene. Despite extensive disruptions of their circulating IGF system after birth, null ALS mice are only slightly growth-retarded and have no obvious defects in carbohydrate metabolism.
Materials and Methods
Inactivation of the ALS Gene.
The ALS replacement vector was constructed by flanking the neomycin phosphotransferase gene of pMC1NEO (Stratagene) with ALS DNA fragments isolated from a mouse genomic library (16). They were a 1.9-kb AccI-AccI fragment corresponding to nucleotides −1,968 to −54 of the mouse ALS gene (throughout the text, numbering is relative to ATG, +1), and a 5.5-kb SacI-SalI fragment extending from nucleotide +2,429 of the mouse ALS gene (Fig. 1). The diphtheria toxin gene was added to select against embryonic stem (ES) cells acquiring G418 resistance by random integration (17).
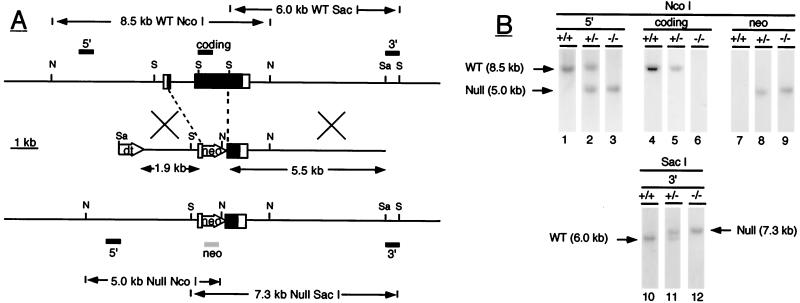
Figure 1.
(A) Inactivation of the ALS gene by homologous recombination. The wild-type ALS gene is shown at the top with exons represented by boxes (coding regions are closed; noncoding regions are open) and with intron and flanking regions represented by horizontal lines. Locations of the restriction endonuclease sites for NcoI (N), SacI (S), and SalI (Sa) are shown by horizontal bars, and positions of the various ALS probes used (5′, coding and 3′) by closed rectangles above the gene. In wild-type DNA, the 5′ and coding ALS probes detect an 8.5-kb NcoI fragment whereas the 3′ probe detects a 6.0-kb SacI fragment. The middle schematic shows the targeting vector containing the ALS genomic fragments and the neomycin phosphotransferase (neo) and diphtheria toxin (dt) genes. Regions of homology between the vector and the wild-type gene are indicated by crossed bars. The null allele produced by homologous recombination is represented at the bottom. In null ALS DNA, the 5′ ALS and neo probes (shown as a shaded rectangle under the null allele) detect a 5.0-kb NcoI fragment whereas the 3′ ALS probe detects a 7.3-kb SacI fragment. (B) Identification of null ALS mice by Southern blotting. Genomic DNA was digested with the restriction enzyme NcoI (lanes 1–9) or SacI (lanes 10–12) and was analyzed by Southern blotting with the indicated DNA probes. The hybridization pattern obtained with each probe is shown for wild-type (+/+), heterozygous (+/−), and null (−/−) ALS mice. DNA fragments corresponding to the wild-type (wt) and null allele (null) are indicated by arrows.
Gene targeting was performed into the 129Sv ES cell line J1 (18). Electroporation, selection, and injection of ES cells into BALB/c blastocysts were performed as described by You-Ten et al. (19). Resulting chimeric males were mated with wild-type BALB/c females, and offspring carrying a null ALS allele were identified by Southern blot analysis.
Southern and Northern Blot Analyses.
Genomic DNA was digested with the restriction endonucleases NcoI or SacI and was analyzed by Southern blotting with DNA probes corresponding to nucleotides −2,223 to −1,983 of the mouse ALS gene (5′ ALS), to nucleotides +163 to +915 of the mouse ALS cDNA (coding ALS), to an ≈500-bp SalI-SacI fragment located immediately downstream of the SacI-SalI fragment used in the targeting vector (3′ ALS), and to nucleotides −251 to +570 of the neomycin gene (neo).
Total RNA was analyzed by Northern blotting using DNA probes corresponding to the internal ALS probe, to nucleotides −22 to +384 of the rat IGF-I cDNA and to nucleotides +409 to +832 of the rat IGFBP-3 cDNA (20, 21). Equality of loading was assessed by reprobing membranes with a low specific activity 18S RNA probe (Ambion, Austin, TX). Signals were quantified by phosphorimaging.
Mouse Care and Experimental Protocol.
Animals were housed in a controlled environment (≈22°C, 14 h light:10 h dark) and were fed a standard laboratory diet (PMI Feeds, St. Louis). For the growth study, mice with two functional ALS alleles (wild-type or +/+), a single null allele (heterozygous or +/−), or two null alleles (null or −/−) were generated by intercrossing heterozygous mice. Animals were weighed every other day from birth until 5 weeks of age, every 5 days between 5 and 8 weeks of age, and weekly between 8 and 10 weeks of age. At 10 weeks of age, tail blood was obtained after sodium pentobarbital anesthesia. Mice were then killed, and tissues were dissected and stored at −80°C. These procedures were approved by the Cornell University Institutional Animal Care Committee.
Affinity Crosslinking Assay.
Plasma samples (1 μl) were incubated overnight at 4°C in 40 μl of PBS with 200,000 cpm of [125I]IGF-I in the absence or presence of 50 ng of recombinant human IGFBP-3 (Celtrix Laboratories, San Jose, CA), followed by crosslinking with disuccinimidyl suberate (6). Complexes were separated by reducing SDS/PAGE and were visualized by autoradiography.
Measurements of Circulating IGFBPs, Hormones, and Metabolites.
Plasma IGFBPs were analyzed by Western ligand blotting using [125I]IGF-I (22). Signals were quantified by phosphorimaging. Plasma IGF-I was assayed by a rat IGF-I RIA (Diagnostic System Laboratories, Webster, TX), plasma insulin by a rat insulin RIA (Linco Research Immunoassay, St. Charles, MO). Concentrations of plasma glucose and nonesterified fatty acids were analyzed by the glucose oxidase method and by the Acyl-CoA synthetase-Acyl-CoA oxidase method, respectively. Intra and interassay coefficients of variation were less than 8% for the RIAs and less than 4% for the metabolic assays.
Statistical Analysis.
Growth data were analyzed by a repeated measure model using the SAS statistical package (SAS Institute, Cary, NC). The mixed model procedure was used with genotype, sex, days, and their interactions as fixed effects and dam, litter, and mouse as random effects. Differences between genotypes at selected times were determined by performing contrasts (+/+ vs. −/− and +/+ vs. +/−), with Bonferroni's adjustment to protect against type I errors. All other data were analyzed by the general linear model (GLM) procedure of SAS using a model accounting for the effects of genotype. When genotype was significant (P < 0.05), individual means were separated by Fisher's least significant differences test.
Results
Derivation of Null ALS Mice.
The strategy used to generate a null ALS mutation is shown in Fig. 1. The targeting vector was designed to replace the coding region of exon 1, intron 1, and over 1,300 bp of exon 2, resulting in the deletion of the first 435 of the 603 amino acid residues of mouse ALS, and 16 of the 19 leucine repeats thought to be essential for ternary complex formation (16).
Cells from a correctly targeted ES colony (ALS9) were injected into blastocysts. Resulting chimeric males were capable of germline transmission when mated to wild-type BALB/c mice. Southern blot analyses of offspring indicated that the null ALS allele had the correct organization. The 5′ and 3′ ALS probes recognized DNA fragments of predicted size for the null allele in heterozygous and null animals (Fig. 1). As expected, the coding ALS probe, corresponding to a region of exon 2 replaced by the neomycin gene, did not hybridize to DNA from null mice. Finally, the neo probe detected a single band, co-migrating with the null ALS allele, indicating that the neomycin gene was present only at the ALS locus.
ALS mRNA and Protein Are Absent in Null Mice.
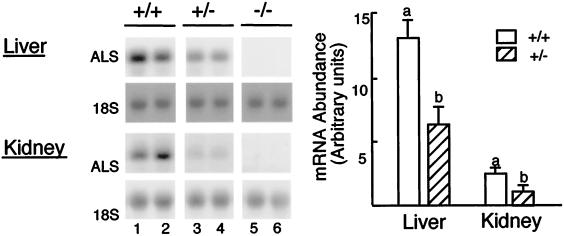
In most species, high ALS gene expression is restricted to postnatal liver (23–25), with the exception of the rat in which expression is also detected in kidney (13). Consistent with this, ALS gene expression was six-fold higher in liver than in kidney in 10-week-old wild-type mice (Fig. 2). ALS mRNA in either tissue was approximately twice as abundant in wild-type mice than in heterozygous mice (P < 0.05) but was completely absent in null mice. These results indicate that ALS synthesis was completely abrogated in null mice.‖
Figure 2.
Effect of ALS genotype on the expression of the ALS gene. (Left) Total RNA was isolated from the liver and kidney of 10-week-old mice. Aliquots (15 μg) from wild-type (+/+, lanes 1 and 2), heterozygous (+/−, lanes 3 and 4), and null (−/−, lanes 5 and 6) ALS mice were analyzed by Northern blotting using the coding ALS probe that detects a unique mRNA of ≈2.2 kb. The signal obtained with the 18S riboprobe is shown for both tissues. (Right) For each tissue, means ± SE of ALS hybridization signals are shown (six animals per genotype). Means with different letters differ at P < 0.05 using GLM followed by Fisher's protected least significant differences analysis.
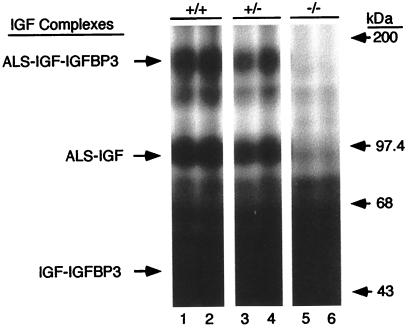
To verify that ALS was absent in null mice, plasma was incubated overnight with [125I]IGF-I and IGFBP-3, followed by crosslinking with disuccinimidyl suberate (Fig. 3). After separation by reducing SDS/PAGE, a signal migrating at ≈150 kDa was visible in plasma of both wild-type and heterozygous mice, consistent with the formation of ternary complexes composed of IGF-I, IGFBP-3, and ALS; this signal was absent in plasma of null mice. A second signal of ≈95 kDa, which represents incomplete crosslinking of ternary complexes [i.e., IGF-I crosslinked to ALS but not to IGFBP-3 (6)], was also detected only in plasma of wild-type and heterozygous animals (Fig. 3). Similar results were obtained when crosslinking was performed without the addition of IGFBP-3, except that the 150-kDa signal was less intense (results not shown). Thus, ALS or a chimeric protein with residual ALS activity is absent in null mice, and their plasma do not support formation of 150-kDa complexes.
Figure 3.
Plasma from null ALS mice is unable to form 150-kDa complexes. Plasma from 10-week-old wild-type (+/+, lanes 1 and 2), heterozygous (+/−, lanes 3 and 4), and null (−/−, lanes 5 and 6) ALS mice were incubated overnight with [125I]IGF-I in the presence of IGFBP-3. After crosslinking with disuccinimidyl suberate, complexes were separated by reducing SDS/PAGE. IGF-I-containing complexes are identified on the left, and positions of the molecular mass standards (in kilodaltons) are given on the right.
Null Mice Suffer from a Modest Growth Retardation After Birth.
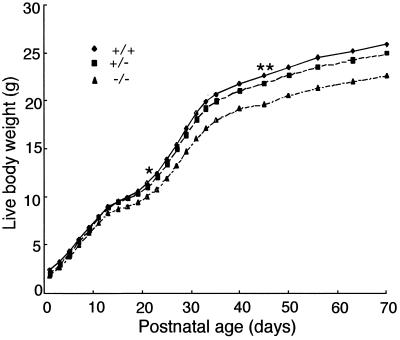
Intercrosses of heterozygous mice yielded offspring (n = 196 from 31 litters) that were distributed according to Mendelian genetics (observed, 48 +/+, 106 +/−, 42 −/− vs. predicted, 49 +/+, 98 +/−, 49 −/−; χ2 test, P > 0.30), indicating that ALS does not affect survival during fetal life. Genotype had no effects on body weight from birth until day 14 of postnatal life (Fig. 4). By day 15, however, null ALS mice tended to be lighter than wild-type mice (P < 0.07). This difference became significant by day 21, and reached a maximum of 13% by day 70 (Fig. 4). A growth deficit was also detected in heterozygous mice after 45 days of postnatal life (P < 0.05) but represented only 4% of wild-type weight by day 70 (Fig. 4). There was no interaction between genotype and sex, or between genotype, sex, and day, indicating that effects of the null genotype were identical in both sexes. We conclude that absence of ALS has no deleterious effects during fetal life but causes a modest growth depression soon after birth.∥
Figure 4.
Null ALS mice grow at reduced rates after birth. Heterozygous mice were intercrossed to generate wild-type (+/+), heterozygous (+/−), and null (−/−) ALS mice (196 offspring from 31 litters). Body weights were recorded from day 1 after birth until 10 weeks of age. Times at which null and heterozygous mice first differed significantly (P < 0.05) from wild-type mice are indicated by an asterisk (*) and a double asterisk (**), respectively.
Finally, we asked whether null animals were reproductively competent. Intercrosses of null mice had litters of slightly smaller size than those of wild-type mice (6.3 vs. 8.7 pups/litter, P < 0.05). The magnitude of this difference did not increase by the time of weaning (6.1 vs. 7.8 pups/litter, P < 0.05), indicating that null females were able to raise their pups. Overall, these data demonstrate that null mice are fertile and able to complete all phases of the reproductive cycle.
The Absence of ALS Reduces Plasma Levels of IGF-I and IGFBP-3.
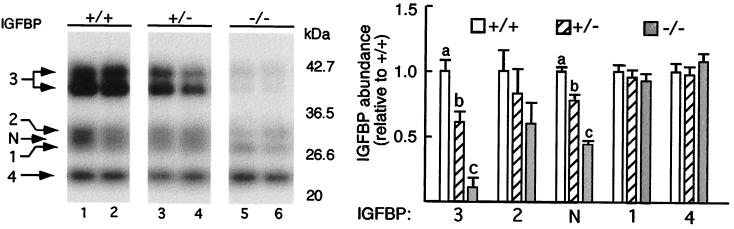
We next assessed the effects of the absence of ALS on plasma concentrations of IGFBPs and IGF-I. Western ligand blot analysis with [125I]IGF-I revealed the presence of five distinct IGFBP species in the plasma of wild-type mice (Fig. 5). The doublet at ≈45 kDa represents glycosylation variants of IGFBP-3 whereas the band at 24 kDa is IGFBP-4. The three bands from 34 to 29 kDa correspond to IGFBP-2, an unknown IGFBP referred to as IGFBP-N, and IGFBP-1 (3, 22). Abundance of the IGFBP-3 doublet was decreased by 40% and 88% in the heterozygous and null ALS mice, respectively.∥ Almost identical reductions were observed across genotypes in the abundance of IGFBP-N, suggesting that it could be a proteolysed form of IGFBP-3, or perhaps, IGFBP-5. In contrast, the abundance of other visible IGFBPs (IGFBP-1, -2, and -4) was not significantly affected by the absence of ALS.
Figure 5.
Plasma IGFBP-3 is decreased in null ALS mice. (Left) Plasma (2 μl) from 10-week-old wild-type (+/+, lanes 1 and 2), heterozygous (+/−, lanes 3 and 4), and null (−/−, lanes 5 and 6) ALS mice were separated by nonreducing SDS/PAGE. After blotting, IGFBPs were detected by incubation with [125I]IGF-I. Signals are identified on the left, and positions of the molecular mass standards (in kilodaltons) are given on the right. IGFBP-N refers to an IGFBP that could not be identified. (Right) Means ± SE of each IGFBP signal, expressed relative to the signal obtained for the wild-type mice, are shown (three to four mice per genotype). For IGFBP-3, the sum of both signals was used in the statistical analysis. Means with different letters differ at P < 0.05 using GLM followed by Fisher's protected least significant differences analysis.
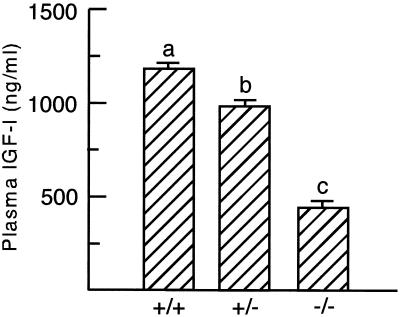
Concentrations of plasma IGF-I were measured by a commercial rat RIA. As previously shown in rat serum (26), the acid-ethanol extraction recommended by the supplier effectively removed IGFBP-3 as well as a major fraction of the lower molecular weight IGFBPs from mouse plasma (data not shown). The concentration of plasma IGF-I was decreased by 17% in the heterozygous mice and by 62% in the null mice∥ (Fig. 6). Identical changes were observed when assaying IGF-I containing fractions after acid gel filtration chromatography of plasma (results not shown). Therefore, we conclude that the absence of ALS causes major reductions in the plasma concentrations of IGF-I and IGFBP-3.
Figure 6.
Concentration of plasma IGF-I is decreased in null ALS mice. Concentrations of IGF-I were assayed from plasma of 10-week-old wild-type (+/+), heterozygous (+/−), and null (−/−) ALS mice. Means ± SE with different letters differ at P < 0.05 using GLM followed by Fisher's protected least significant differences analysis (eight mice per genotype).
Synthesis of IGFBP-3 and IGF-I in Liver Is Not Reduced in Null Mice.
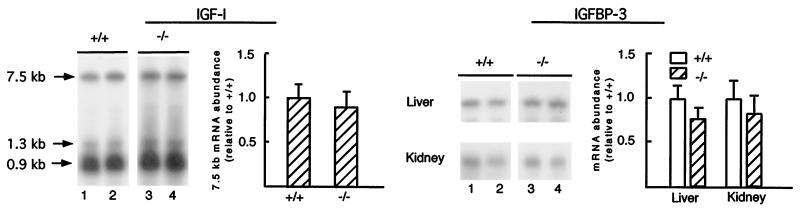
To determine whether the reduced plasma concentrations of IGF-I and IGFBP-3 in null ALS mice results from increased turnover or decreased synthesis, abundance of IGF-I and IGFBP-3 mRNA was measured in liver and kidney. Liver produces at least 70% of circulating IGF-I in the mouse, and liver and kidney are major sites of IGFBP-3 synthesis (27–29). Null ALS mice did not have lower abundance of IGF-I mRNA in liver or IGFBP-3 mRNA in liver and kidney∥ (Fig. 7). Consistent with the ligand blot data, expression of IGFBP-1, -2, -4, and -5 in liver and kidney also was not affected by the ALS genotype (data not shown). Overall, these data suggest that accelerated turnover is the primary factor responsible for the reduction of circulating IGF-I and IGFBP-3 in null mice.
Figure 7.
Expression of IGF-I and IGFBP-3 mRNAs is not altered in null ALS mice. Total RNA (15 μg) from the liver and kidney of 10-week-old wild-type (+/+, lanes 1 and 2) and null (−/−, lanes 3 and 4) ALS mice were analyzed by Northern blotting using an IGF-I (Left) or IGFBP-3 (Right) probe. For each probe, signals were normalized to the signal obtained with the 18S riboprobe (shown in Fig. 2). Means ± SE (six mice per genotype) are presented relative to the signal obtained for the wild-type animal. Means did not differ at P < 0.05 using GLM.
The Absence of ALS Has No Effects on Indices of Carbohydrate Metabolism.
Decreased formation of ternary complexes during tumor-induced hypoglycemia is thought to be involved in causing decreased concentrations of plasma glucose and insulin (3, 4, 30). To determine whether the absence of ALS led to changes in carbohydrate metabolism, blood was obtained from fed mice at 10 weeks of age. Wild-type and null mice had similar concentrations of plasma glucose (231 ± 4 vs. 224 ± 5 mg/dl), insulin (0.73 ± 0.10 vs. 0.69 ± 0.10 ng/ml), and nonesterified fatty acids (412 ± 28 vs. 447 ± 29 μM). These data suggest that, under basal conditions, carbohydrate metabolism and insulin action were not affected by the absence of ALS.∥
Discussion
Onset of ALS synthesis in liver around the time of birth represents one of the last events in the development of the circulating IGF system. Until then, plasma IGFs circulate as IGF:IGFBP complexes of ≈50 kDa (3, 5). Secretion of ALS into the circulation drives most of the plasma IGFs into ternary complexes of 150 kDa (3–5). GH-deficient models have shown that ALS synthesis and formation of ternary complexes are both GH-dependent (9, 11). However, because GH regulates so many additional processes (12), the functional significance of ALS and ternary complexes to normal growth and development cannot be deduced from these experiments. To address these issues, we have created a strain of mice in which the ALS gene is inactivated. This disruption completely abrogated synthesis of ALS as shown by the absence of mRNA in null mice and by the inability of their plasma to support ternary complex formation.
Baxter and coworkers showed that ALS binds only to IGFBP-3 or IGFBP-5 when they are occupied by IGFs (6, 7). In the present study, plasma concentrations of IGF-I and IGFBP-3 were decreased by 62 and 88% in the null ALS mice whereas levels of IGFBPs that do not interact with ALS (i.e., IGFBP-1, -2, and -4) were unaffected. These reductions likely reflect accelerated turnover of plasma IGF-I and IGFBP-3 because they occurred without any changes in their hepatic or renal expression. These findings are consistent with the shorter half-lives of IGFs when not sequestered in ternary complexes (2–4). Moreover, they show that without ALS, induction of IGF-I and IGFBP-3 synthesis after birth would only cause a modest increase in their plasma concentrations (29, 31). Thus, ALS is an essential component of the circulating IGF system.
Study of heterozygous animals yielded further insight on the regulation of ALS. First, abundance of ALS mRNA in their kidney and liver was only 50% of that of wild-type mice, indicating that normal levels of gene expression requires transcription from both alleles. Second, this reduction in gene expression resulted in lower plasma levels of IGF-I and IGFBP-3. Therefore, efficient capture of IGFs into ternary complexes depends on a 2- to 3-fold molar excess of ALS relative to the concentration of IGF, a reflection of the low affinity of ALS for binary complexes of IGF and IGFBP-3 (4, 7, 32). As a consequence, concentration of plasma IGFs can be altered simply by changing ALS synthesis.
As expected, a growth deficit became obvious at 3 weeks of age in the null ALS mice when liver normally increases its output of IGF-I and ALS in response to GH (23, 31, 32). However, this phenotype is surprisingly modest, given the important role postulated for plasma IGF-I by the somatomedin hypothesis in regulating postnatal growth and late developmental processes such as lactation (1, 8, 33). Three explanations are suggested. First, because of its occurrence in binary complexes, the total amount of plasma IGF-I reaching target tissues may not have been reduced in null mice (2, 3). Second, locally produced IGF-I may assume a predominant role in carrying the GH-dependent and independent effects of IGF-I. In support of this view, abrogation of IGF-I synthesis only in liver resulted in a reduction in the concentration of plasma IGF-I comparable to the one we observed in null ALS mice, but did not alter postnatal growth (27, 28). These mice, however, are expected to have most of their plasma IGF-I in ternary complexes. They also have a six-fold elevation in plasma GH, resulting in liver hypertrophy and perhaps compensatory induction of IGF-I transcription in other tissues such as adipose tissue and the growth plate (27). In null ALS mice, increased GH secretion is unlikely because liver size (results not shown) and hepatic IGF-I gene expression are both normal. Finally, it is possible that the mixed genetic background of our mice attenuated the null ALS phenotype.
Near normal growth under idealized conditions does not mean that liver-derived IGF-I and ternary complexes are dispensable under all circumstances. A modified somatomedin hypothesis that would accommodate findings in null ALS mice and in mice with inactivation of the liver IGF-I gene is one in which the primary function of liver is to supply the IGF-I needed to respond to various challenges. In this model, ALS plays a critical role by capturing liver-derived IGF-I into long-lived ternary complexes. This model would predict that null ALS mice with limited retention of liver-derived IGF-I would not respond as well to GH therapy or would have greater muscle wasting after endotoxin exposure (34, 35). In support of this hypothesis, IGF-I therapies are more effective when ternary complex formation is improved by co-administration of GH or IGFBP-3 (36, 37).
Incorporation into ternary complexes is likely to be important against unregulated metabolic and mitogenic effects of IGFs. Containment of metabolic effects may be particularly important for IGF-II whose signaling via the insulin receptor is required for complete fetal growth and development (38). This signaling, however, must be restrained after birth when the insulin receptor becomes responsible for metabolic regulation. If not, chronic hypoglycemia and hypoinsulinemia would likely develop, as shown by individuals suffering from non-islet tumor-induced hypoglycemia (4, 30). The main reason why the null ALS mice do not suffer from similar abnormalities is that the IGF-II gene is silenced in most rodent tissues soon after birth, resulting in virtually no circulating IGF-II (39). This is in contrast to most other mammals, which have plasma concentrations of IGF-II exceeding that of IGF-I by 1- to 3-fold (8). Therefore, postnatal expression of ALS and ternary complex formation may be important in restraining the hypoglycemic actions of IGF-II, an hypothesis that can be tested by intercrossing null ALS and IGF-II overexpressing mice (40). Finally, protection afforded by ALS is likely to extend to the mitogenic effects of IGFs as well. Evidence supporting this hypothesis includes increased incidence of tumors in transgenic mice overexpressing IGF-II (40), defects promoting higher concentrations of IGF-II in some cancer cells (41, 42), and the positive association between concentrations of plasma IGF-I and incidence of prostate and breast cancers in human populations (43, 44). Although these studies do not establish a cause and effect relationship, they suggest that, without ALS, excessive stimulation of proliferation could increase the incidence of cancers.
In summary, absence of ALS causes dramatic disruptions to the postnatal circulating IGF system but, consistent with a predominant role for locally synthesized IGF-I on growth (27, 28), only modest effects on that process. Our results also suggest that, without ALS to safely store them, liver-derived IGF-I would be less effective in promoting specific responses such as the acceleration of growth after GH therapy. The null ALS mouse model should be useful for testing the importance of circulating IGFs, particularly when combined with other transgenic models of the IGF system (27, 40).
Acknowledgments
We thank Drs. S. Chernausek (University of Cincinnati) and D. Leroith (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD) for performing the acid gel permeation chromatography. This work was supported by National Institutes of Health Grant DK-51624 (to Y.R.B.) and by the Cornell University Agricultural Experiment Station.
Abbreviations
- IGF
insulin-like growth factor
- GH
growth hormone
- IGFBP
IGF-binding protein
- ALS
acid labile subunit
- ES
embryonic stem
- GLM
general linear model
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120172697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120172697
Identical results were observed in a different line of null ALS mice derived from a second correctly targeted ES cell (ALS12).
References
- 1.Stewart C E H, Rotwein P. Physiol Rev. 1996;76:1005–1026. doi: 10.1152/physrev.1996.76.4.1005. [DOI] [PubMed] [Google Scholar]
- 2.Jones J I, Clemmons D R. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 3.Rechler M M. Vitam Horm (New York) 1993;47:1–114. doi: 10.1016/s0083-6729(08)60444-6. [DOI] [PubMed] [Google Scholar]
- 4.Baxter R C. Horm Res. 1994;42:140–144. doi: 10.1159/000184186. [DOI] [PubMed] [Google Scholar]
- 5.Ooi G T, Boisclair Y R. In: Contemporary Endocrinology: The IGF System. Rosenfeld R, Roberts C Jr, editors. Totowa, NJ: Humana; 1999. pp. 111–139. [Google Scholar]
- 6.Baxter R C, Martin J L. Proc Natl Acad Sci USA. 1989;86:6898–6902. doi: 10.1073/pnas.86.18.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twigg S M, Baxter R C. J Biol Chem. 1998;273:6074–6079. doi: 10.1074/jbc.273.11.6074. [DOI] [PubMed] [Google Scholar]
- 8.Daughaday W H, Rotwein P. Endocr Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- 9.Gargosky S E, Tapanainen P, Rosenfeld R G. Endocrinology. 1994;134:2267–2276. doi: 10.1210/endo.134.5.7512499. [DOI] [PubMed] [Google Scholar]
- 10.Bichell D P, Kikuchi K, Rotwein P. Mol Endocrinol. 1992;6:1899–1908. doi: 10.1210/mend.6.11.1480177. [DOI] [PubMed] [Google Scholar]
- 11.Ooi G T, Cohen F J, Tseng L Y-H, Rechler M M, Boisclair Y R. Mol Endocrinol. 1997;11:997–1007. doi: 10.1210/mend.11.7.9942. [DOI] [PubMed] [Google Scholar]
- 12.Carter-Su C, Schwartz J, Smit L S. Annu Rev Physiol. 1996;58:187–207. doi: 10.1146/annurev.ph.58.030196.001155. [DOI] [PubMed] [Google Scholar]
- 13.Chin E, Zhou J, Dai J, Baxter R C, Bondy C A. Endocrinology. 1994;134:2498–2504. doi: 10.1210/endo.134.6.7515002. [DOI] [PubMed] [Google Scholar]
- 14.Xu S, Cwyfan-Hughes S C, Van der Stappen J W J, Sansom J, Burton J L, Donnelly M, Holly J M P. J Clin Endocrinol Metab. 1995;80:2940–2945. doi: 10.1210/jcem.80.10.7559878. [DOI] [PubMed] [Google Scholar]
- 15.Cwyfan Hughes S C, Mason H D, Franks S, Holly J M P. J Endocrinol. 1997;155:R1–R4. doi: 10.1677/joe.0.155r001. [DOI] [PubMed] [Google Scholar]
- 16.Boisclair Y R, Seto D, Hsieh S, Hurst K R, Ooi G T. Proc Natl Acad Sci USA. 1996;93:10028–10033. doi: 10.1073/pnas.93.19.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCarrick J W, Parnes J R, Seong R H, Solter D, Knowles B B. Transgenic Res. 1993;2:183–190. doi: 10.1007/BF01977348. [DOI] [PubMed] [Google Scholar]
- 18.Li E, Bestor T H, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 19.You-Ten K E, Muise E S, Itie A, Michaliszyn E, Wagner J, Jothy S, Lapp W S, Tremblay M L. J Exp Med. 1997;186:683–693. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts C T, Jr, Lasky S R, Lowe W L, Jr, Seaman W T, LeRoith D. Mol Endocrinol. 1987;1:243–248. doi: 10.1210/mend-1-3-243. [DOI] [PubMed] [Google Scholar]
- 21.Shimasaki S, Koba A, Mercado M, Shimonaka M, Ling N. Biochem Biophys Res Commun. 1989;165:907–912. doi: 10.1016/s0006-291x(89)80052-x. [DOI] [PubMed] [Google Scholar]
- 22.Dai Z, Xing Y, Boney C M, Clemmons D R, D'Ercole A J. Endocrinology. 1994;135:1316–1327. doi: 10.1210/endo.135.4.7523094. [DOI] [PubMed] [Google Scholar]
- 23.Dai J, Baxter R C. Endocrinology. 1994;135:2335–2341. doi: 10.1210/endo.135.6.7527331. [DOI] [PubMed] [Google Scholar]
- 24.Delhanty P, Baxter R C. Biochem Biophys Res Commun. 1996;227:897–902. doi: 10.1006/bbrc.1996.1602. [DOI] [PubMed] [Google Scholar]
- 25.Rhoads R P, Greenwood P L, Bell A W, Boisclair Y R. Endocrinology. 2000;141:1425–1433. doi: 10.1210/endo.141.4.7425. [DOI] [PubMed] [Google Scholar]
- 26.Crawford B A, Martin J L, Howe C J, Handelsman D J, Baxter R C. J Endocrinol. 1992;134:169–176. doi: 10.1677/joe.0.1340169. [DOI] [PubMed] [Google Scholar]
- 27.Yakar S, Liu J-L, Stannard B, Butler A, Accili D, Sauer B, LeRoith D. Proc Natl Acad Sci USA. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sjogren K, Liu J-L, Blad K, Skrtic S, Vidal O, Wallenius V, LeRoith D, Tornell J, Isaksson O G P, Jansson J-L, Ohlsson C. Proc Natl Acad Sci USA. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albiston A L, Herington A C. Endocrinology. 1992;130:497–502. doi: 10.1210/endo.130.1.1370153. [DOI] [PubMed] [Google Scholar]
- 30.Baxter R C, Holman S R, Corbould A, Stranks S, Ho P J, Braund W. J Clin Endocrinol Metab. 1995;80:2700–2708. doi: 10.1210/jcem.80.9.7545698. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi K, Bichell D P, Rotwein P. J Biol Chem. 1992;267:21505–21511. [PubMed] [Google Scholar]
- 32.Baxter R C, Dai J. Endocrinology. 1994;134:848–852. doi: 10.1210/endo.134.2.7507839. [DOI] [PubMed] [Google Scholar]
- 33.Etherton T D, Bauman D E. Physiol Rev. 1998;78:745–761. doi: 10.1152/physrev.1998.78.3.745. [DOI] [PubMed] [Google Scholar]
- 34.Clark R G, Mortensen D L, Carlsson L M S. Endocrine. 1995;3:297–304. doi: 10.1007/BF03021409. [DOI] [PubMed] [Google Scholar]
- 35.Frost R A, Lang C H. Curr Opin Clin Nutr Metab Care. 1998;1:195–204. doi: 10.1097/00075197-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kupfer S R, Underwood L E, Baxter R C, Clemmons D R. J Clin Invest. 1993;91:391–396. doi: 10.1172/JCI116212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagi C M, DeLeon E, Brommage R, Rosen D, Sommer A. Calcif Tissue Int. 1995;57:40–46. doi: 10.1007/BF00298995. [DOI] [PubMed] [Google Scholar]
- 38.Louvi A, Accili D, Efstratiadis A. Dev Biol. 1997;189:33–48. doi: 10.1006/dbio.1997.8666. [DOI] [PubMed] [Google Scholar]
- 39.Brown A L, Graham D E, Nissley S P, Hill D J, Strain A J, Rechler M M. J Biol Chem. 1986;261:13144–13150. [PubMed] [Google Scholar]
- 40.Rogler C E, Yang D, Rossetti L, Donohoe J, Alt E, Chang C J, Rosenfeld R, Neely K, Hintz R. J Biol Chem. 1994;269:13779–13784. [PubMed] [Google Scholar]
- 41.Toretsky J A, Helman L J. J Endocrinol. 1996;149:367–372. doi: 10.1677/joe.0.1490367. [DOI] [PubMed] [Google Scholar]
- 42.De Souza A T, Yamada T, Mills J J, Jirtle R L. FASEB J. 1997;11:60–67. doi: 10.1096/fasebj.11.1.9034167. [DOI] [PubMed] [Google Scholar]
- 43.Rosen C J, Pollak M. Trends Endocrinol Metab. 1999;10:136–141. doi: 10.1016/s1043-2760(98)00126-x. [DOI] [PubMed] [Google Scholar]
- 44.Chan J M, Stampfer M J, Giovannucci E, Gann P H, Ma J, Wilkinson P, Hennekens C H, Pollak M. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]