Abstract
The efficacy of a new recombinant subunit West Nile virus (WNV) vaccine candidate was determined in a hamster model of meningoencephalitis. Groups of hamsters were immunized subcutaneously with a WNV recombinant envelope protein (80E) with or without WNV non-structural protein 1 (NS1) mixed with adjuvant or adjuvant alone. At 2 weeks, 6 months, and 12 months after two immunizations at 4 week intervals with the respective immunogens, groups of animals were challenged via the intraperitoneal route with a virulent strain of WNV. The two recombinant antigen preparations gave similar results; hamsters in both groups had a strong antibody response following immunization, and none of the animals became ill or developed detectable viremia after challenge with WNV at 2 weeks or 6 months post booster vaccination. In contrast, , mortality among the control animals at 2 weeks post booster challenge was 73%, and at 6 months post booster, the mortality was 53% among the control animals. When challenged 12 months after the booster vaccination, a low level viremia was detected in some of the vaccinated hamsters, and one hamster became sick, but recovered. In contrast, all of the control animals that received adjuvant only developed a viremia, and the mortality rate was 77%. These results with the recombinant subunit WNV vaccine are very encouraging and warrant further animal studies to evaluate its potential use to protect humans against WNV disease.
1. Introduction
Since the first recognition of West Nile virus (WNV) in New York City in 1999, the virus has spread rapidly throughout North America (United States, Canada and Mexico) [1–3]. As the virus has extended its distribution, the number of reported human cases and the public health importance of WNV infection have also increased. It now appears that WNV is permanently established in North America and that it will probably continue to spread into Central and South America.
Based on retrospective serosurveys conduced in New York City in 1999 and 2000, it was found that about 20% of persons infected with WNV develop symptomatic illness, most commonly a flu-like illness referred to as “West Nile fever” [1]. From these studies, it was estimated that about 1 in 150 human infections with WNV in the United States will develop severe neurologic symptoms. In recent epidemics of WNV meningoencephalitis, the fatality: case ratios of this form of the virus infection have ranged from 4–14% [1]. The risk of severe or fatal WNV infection is higher in the elderly, immunosuppressed persons and diabetics. Because of its increasing public health importance in North America, there is considerable interest in developing a vaccine for the prevention of WNV disease in humans.
This paper reports the results of studies on the efficacy of a new recombinant subunit West Nile vaccine candidate, developed by Hawaii Biotech, Inc., in protecting against severe WNV infection in a golden hamster model of meningoencephalitis [4].
2. Materials and Methods
2.1. Animals
The animals used in these studies were 8–10 week old female Syrian golden hamsters (Mesociacetus auratus) obtained from Harlan Spraque Dawley, Indianapolis, IN. Animals were cared for in accordance with the guidelines of the Committee on Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council) under an animal use protocol approved by the University of Texas Medical Branch. All work with infected animals was carried out in an AAALAC accredited biosafety level-3 facility.
2.2. Description of vaccines and vaccination schedule
The production and purification of the vaccine antigens are described in detail in the accompanying paper [5]. Briefly, West Nile virus carboxy-truncated envelope protein (80E) and nonstructural protein 1 (NS1) were produced in an insect cell (Drosophila) expression system. The proteins were purified by immunoaffinity chromatography (IAC), using monoclonal antibodies that are flavivirus envelope protein group specific (for the 80E) or flavivirus NS1 group specific (for NS1). For vaccination of hamsters, 1 or 10 μg of 80E +/− 1 μg of NS1 (or 1 μg of NS1 alone in one experiment) was used as the immunizing dose per animal. The antigens were mixed with ISCOMATRIX® adjuvant (12 μg; CSL Ltd., Parkville, Melbourne, Victoria, Australia). In addition, the adjuvant control vaccine was formulated to include “mock” antigen. This material was prepared by subjecting culture supernatants from induced Drosophila cells transformed with plasmids lacking the genes encoding the specific antigens to the same purification schemes used for the 80E protein. The purpose of including this material with adjuvant was to control for any possible non-specific immunostimulatory effects of potential contaminants from the cell cultures co-purified with the antigens. An amount of this “mock” antigen equivalent to what would be present in 10 μg of 80E was used per dose. Each hamster was inoculated twice via the subcutaneous route with 0.5 ml at 4 week intervals.
2.3. Virus used to challenge the animals
At the prescribed times after the second immunization, hamsters were challenged intraperitoneally with 104 plaque forming units (PFU) units of West Nile virus strain NY 385–99. This dose was previously shown to cause a mortality rate 50% or more in 8 to 10 week old hamsters. The virus strain was originally isolated from a dead bird at the Bronx Zoo during the 1999 epizootic in New York City and had been passaged twice in Vero cells [4]. After inoculation with WNV, 6 hamsters in each group were bled daily (200 μL from retroorbital sinus complex) for 6 consecutive days to determine the level and duration of viremia and the antibody response. All animals were examined daily for signs of illness or death, and on day 30 after challenge, all of the surviving animals were euthanized.
2.4. Virus titration and antibody determinations
Blood samples from the hamsters were processed differently, depending on their use. Whole blood for virus titration and neutralization tests was collected from the retroorbital sinus (100 μL) and was diluted 1:10 in phosphate-buffered saline, pH 7.4 (PBS), containing 25% heat-inactivated fetal bovine serum; these samples were frozen at –85°C immediately after collection, until tested. Blood for hemagglutination inhibition (HI) and complement fixation (CF) antibody determinations (100 μL) was obtained by the same method and was diluted 1:10 in saline without buffer. This suspension was centrifuged to sediment the blood cells, and the supernatant was aspirated and stored at –20°C until tested for HI antibody.
Serial dilutions of hamster blood, ranging from 10–1 to 10–7 were tested for virus in 24-well microplate cultures of Vero cells. Four microplate wells were inoculated with 100 μl of each dilution. A double overlay system was used, consisting of 2% Noble agar and minimal essential medium with Earle’s salts (MEM) containing 2% fetal bovine serum, 0.21% NaHCO3, 2 mM L-glutamine, 100 units/mL of penicillin, 100 μg/mL of streptomycin and 1.5% DEAE-dextran. The second overlay contained neutral red. Plaques were read on the fourth day after inoculation; virus titers were calculated as the number of plaque forming units (pfu) per 0.1 ml of blood.
Serum antibodies to WNV were measured by HI, complement-fixation (CF) and plaque reduction neutralization tests (PRNT), as described before [6, 7]. Antigens for the HI and CF tests were prepared from brains of newborn mice inoculated intracerebrally with WNV; infected brains were treated by the sucrose-acetone extraction method [7]. Hamster sera were tested by HI at serial two-fold dilutions from 1:20 to 1:5,120 at pH 6.6 with 4 units of antigen and a 1:200 dilution of goose erythrocytes. CF tests were performed by a microtechnique [7] with two full units of guinea pig complement and antigen titers ≥1:32. Titers were recorded as the highest dilutions giving +3 or +4 fixation of complement on a scale of 0 to +4.
PRNT tests on hamster sera were performed by a previously described technique [6] in 24-well, Vero microplate cultures, using a fixed inoculum of WNV (~100 pfu) against varying serum dilutions (1:20 to 1:20,480). Hamster sera were diluted in PBS containing 10% fresh guinea pig serum. The virus inoculum was mixed with an equal volume of each serum dilution; and the mixture was incubated overnight at 4°C. The following day, 50 μL of the serum-virus mixture was injected into Vero microplate cultures, using two wells per serum dilution. Virus plaques were read 4 days later; ≥90% plaque reduction was used as the endpoint.
3. Results
3.1. Experiment 1. Protection with 80E, NS1, or 80E + NS1
As shown in Table 1, in the first experiment, groups of 15 hamsters each were inoculated with either the WN vaccine or the mock antigen, and a booster was given 4 weeks later. Two weeks after the booster, blood samples were obtained to determine antibody titers, and the animals were challenged with wild type WN virus. As shown in Figure 1, the HI antibody titers were about the same for the 4 vaccinated groups (#3 through #6), with a reciprocal geometric mean titer (GMT) of about 250. Groups 1 and 2 (adjuvant control and NS1 only) were negative (titers <1:20). None of the animals that received 80E vaccine showed any signs of illness during the 30 day observation period thus demonstrating 100% protection (Table 2). In addition, partial protection was obtained with NS1 (group #2) alone (with adjuvant) as indicated by 87% survival rate as opposed to a 47% rate for the animals (group #1) that received mock adjuvant/antigen. Also, none of the 80E vaccinated animals developed a detectable viremia (Figure 2). In contrast, the adjuvant control group #1 had a pattern of viremia typical of naïve animals (4). Group 2 (NS1 only vaccinated hamsters) had a lower overall level of viremia, which was cleared earlier than the group 1 animals. These results support the protection induced by the 80E WN vaccine and the partial protection afforded by the WN NS1.protein.
Table 1.
Experimental design for the evaluation of a West Nile candidate vaccine 80E with or without the West Nile NS1 protein in groups of hamsters a
| Group no. | # of animals | 80E dose (μg) | NS1 dose (μg) | “mock” antigenb |
|---|---|---|---|---|
| 1 | 15 | 0 | 0 | + |
| 2 | 15 | 0 | 1 | − |
| 3 | 15 | 1 | 0 | − |
| 4 | 15 | 1 | 1 | − |
| 5 | 15 | 10 | 0 | − |
| 6 | 15 | 10 | 1 | − |
All vaccines contained 12 μg of ISCOMATRIX® adjuvant per dose. Hamsters were vaccinated twice, subcutaneously, at a 4 week interval, and then challenged 2 weeks later.
An amount of “mock” antigen equivalent to what would be present in 10 μg of 80E was used.
Fig. 1.
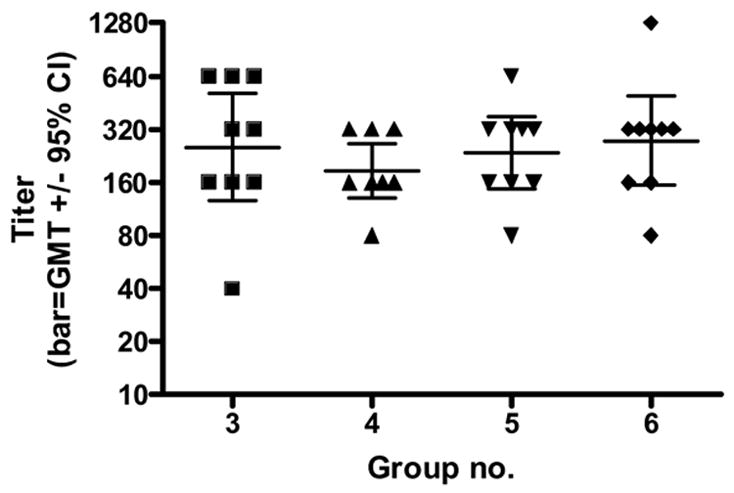
West Nile hemagglutination inhibition pre-challenge antibody titers for hamsters based on results for individual animals of each group. Tests of significance (p <0.05) were performed between groups using an unpaired t test with the aid of a commercially available statistical program (GraphPad Prizm). No significant differences were found.
Table 2.
Protective efficacy of a West Nile candidate vaccine in golden hamsters based on challenge with wild type West Nile virus
| Hamsters Group no. | West Nile immunogens | Hamsters # Survivors/totala | Hamsters % Survivala |
|---|---|---|---|
| 1 | adjuvant/mock antigenb | 7/15 | 47 |
| 2 | 1 μg NS1 | 13/15c | 87 |
| 3 | 1 μg 80E | 15/15d | 100 |
| 4 | 1 μg 80E+1 μg NS1 | 15/15d | 100 |
| 5 | 10 μg 80E | 15/15d | 100 |
| 6 | 10 μg 80E+1 μg NS1 | 15/15d | 100 |
30 days post challenge.
12 μg of ISCOMATRIX® adjuvant per dose present in all groups; “mock antigen” = equivalent of 10 μg of 80E purified by IAC from an induced “mock” 80E transformed S2 cell line, i.e., S2 cells transformed with plasmid DNA without the prM80E insert [5].
p value = 0.022 relative to group 1 (Fisher exact probability test).
p value = 0.0011 relative to group 1 (Fisher exact probability test).
Fig. 2.
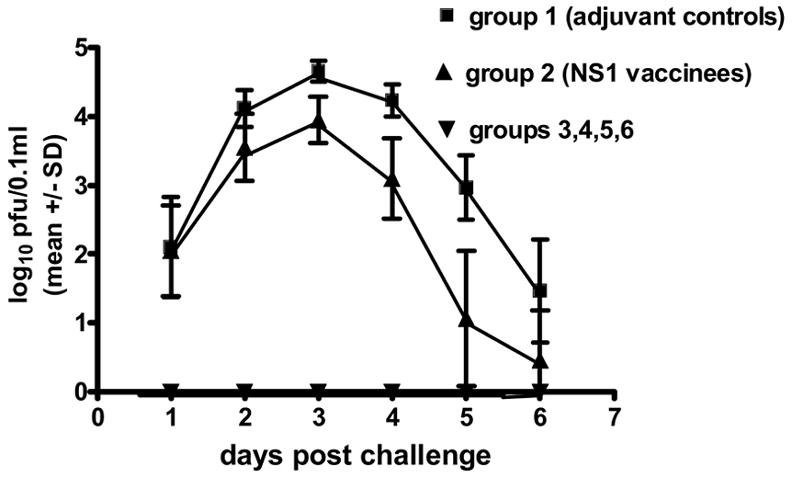
West Nile viremia mean titers (log10 pfu/0.1ml) for each group of hamsters for each day post challenge by intraperitoneal inoculation with 104 TCID50 of West Nile virus. Groups 3–6 had no detectable viremia in any animal. Limit of detection: 100.7 pfu/0.1ml. Tests of significance (p <0.05) were performed between groups 1 and 2 at each day post challenge using an unpaired t test with the aid of a commercially available statistical program (GraphPad Prizm). Differences were significant at days 2–6.
3.2. Experiment 2. Durability of Protection
The WN vaccination groups of animals evaluated in experiment 2 and their respective times of challenge post booster vaccination are presented in Table 3. As shown in Table 4, the HI, CF, and PRNT antibody titers remained steady through 6 months post booster vaccination. At 12 months post booster, the HI and CF antibody titers have decreased substantially. However, the PRNT titers remain as high or higher than at the earlier time points. At 2 weeks, 6 months, or 12 months post booster, groups of 15 animals were challenged and observed for signs of illness and mortality for 30 days. These results demonstrated that the WN recombinant subunit vaccine induced effective durable protection against lethal encephalitis for at least 12 months post booster vaccination (Table 5). In addition, these clinical observations for the vaccinated animals were supported by the absence of a detectable viremia in any of the vaccinated groups when challenged either 2 weeks or 6 months post booster (Fig. 3A), whereas the adjuvant control groups again had patterns of viremia typical of challenged naïve animals. When challenged 12 months post booster vaccination (Fig. 3B), the vaccinated animals showed a low level of viremia, with a mean of about 1 log of virus/0.1ml blood at the peak (3–4 days post challenge), compared to the control group which peaked at about 5 logs of virus/0.1ml of blood on day 3 post challenge.
Table 3.
Experimental design for assessing the durability of the efficacy of a West Nile candidate vaccine vaccine 80E with or without the West Nile NS1 protein in groups of hamsters a
| Group no. | # of animals | 80E dose (μg) | NS1 dose (μg) | Challenge time |
|---|---|---|---|---|
| 1 | 15 | 0 | 0 | 2 weeks post booster |
| 2 | 15 | 1 | 0 | 2 weeks post booster |
| 3 | 15 | 0 | 0 | 6 months post booster |
| 4 | 15 | 1 | 0 | 6 months post booster |
| 5 | 15 | 1 | 1 | 6 months post booster |
| 6 | 15 | 0 | 0 | 12 months post booster |
| 7 | 15 | 1 | 0 | 12 months post booster |
| 8 | 15 | 1 | 1 | 12 months post booster |
All vaccines contained 12 μg of ISCOMATRIX® adjuvant per dose. Hamsters were vaccinated twice, subcutaneously, at a 4 week interval, and then challenged 2 weeks, 6 months, or 12 months later. Groups 1, 3, and 6 vaccines contained an amount of “mock” antigen equivalent to what would be present in 2 μg of 80E.
Table 4.
Summary of West Nile post booster vaccination antibody titers in hamsters prior to challenge with wild type West Nile virus
| Group # | Vaccine | Time of Assay Post Booster Vaccination | HI Titera | CF Titera | PRNT90 Titera |
|---|---|---|---|---|---|
| 1 | Adjuvant Control | 2 weeks | <20 | <10 | <10 |
| 2 | 1 μg 80E | 2 weeks | 113 (87–147) | 57 (44–73) | 84 (37–183) |
| 3 | Adjuvant Control | 6 months | <20 | <10 | <20 |
| 4 | 1 μg 80E | 6 months | 133 (98–181) | 58 (38–89) | 211 (154–290) |
| 5 | 1 μg 80E + 1 μg NS1 | 6 months | 121 (91–161) | 101 (67–151) | 254 (186–348) |
| 6 | Adjuvant Control | 12 months | <10 | <10 | <20 |
| 7 | 1 μg 80E | 12 months | 38 (19–76) | 10 (7–15) | 266 (159–444) |
| 8 | 1 μg 80E + 1 μg NS1 | 12 months | 26 (14–48) | 10 (6–16) | 390 (230–663) |
Geometric mean (GMT) hemagglutination inhibition (HI), complement fixation (CF) and neutralizing antibody (PRNT) titers for individual animals (lower 95% CI of GMT-upper 95% CI of GMT; N=14 for groups 2,8, N=15 all other groups)
Table 5.
Durability of the protective efficacy of a West Nile candidate vaccine in golden hamsters based on challenge with wild type West Nile virus
| Vaccination Group | Challenge Time | No. Survivors/Total Infected | % Survival | No. Sick but Recovered |
|---|---|---|---|---|
| Adjuvant control | 2 weeks post boost | 4/15 | 27 | 4 |
| 1μg 80E | 2 weeks post boost | 15/15 | 100 | 0 |
| Adjuvant control | 6 months post boost | 7/15 | 47 | 7 |
| 1μg 80E | 6 months post boost | 15/15 | 100 | 0 |
| 1μg 80E + 1 μg NS1 | 6 months post boost | 15/15 | 100 | 0 |
| Adjuvant control | 12 months post boost | 5/15 | 33 | 5 |
| 1μg 80E | 12 months post boost | 14a/15 | 93 | 1 |
| 1μg 80E + 1 μg NS1 | 12 months post boost | 14b/15 | 93 | 0 |
The one animal that died had severe malocclusions which prevented eating properly. No specific clinical signs of WN disease were present prior to death.
One animal was found dead in its cage. No specific clinical signs of WN disease were noticed prior to death.
Fig. 3.
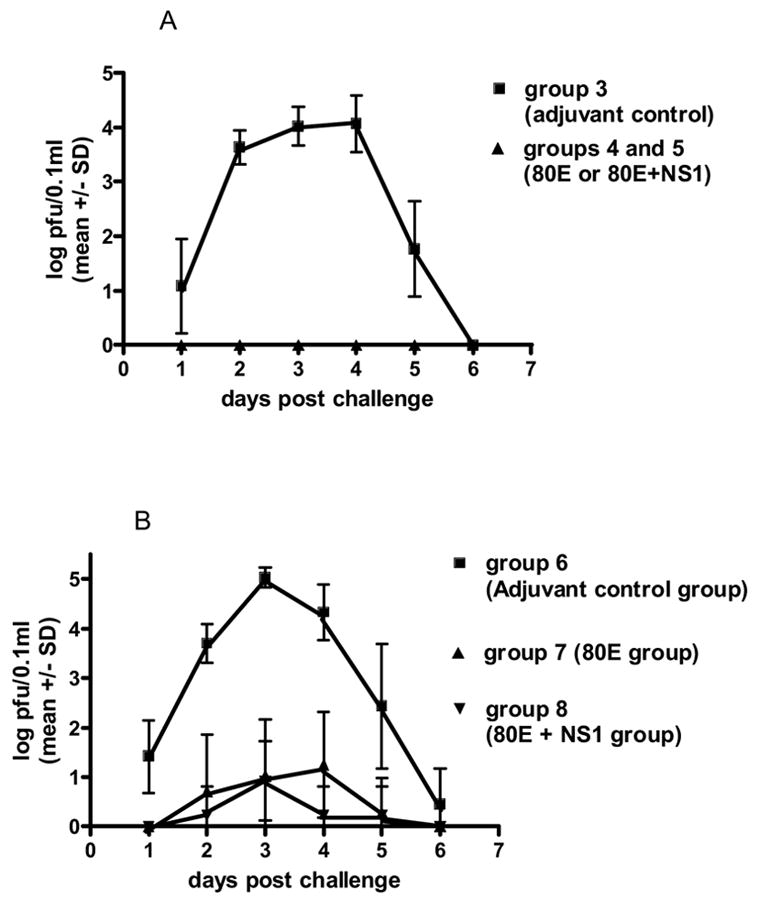
West Nile viremia mean titers (log10 pfu/0.1ml) for each group of hamsters for each day post challenge by intraperitoneal inoculation with 104 TCID50 of West Nile virus. Limit of detection: 100.7 pfu/0.1ml. A. Challenge of hamsters at 6 months post booster vaccination. (Challenge at 2 weeks post booster vaccination yielded very similar results for the adjuvant control and 80E vaccinee groups.) B. Challenge of hamsters at 12 months post booster vaccination. Tests of significance (p <0.05) were performed between groups at each day post challenge using an unpaired t test with the aid of a commercially available statistical program (GraphPad Prizm). Differences were significant between group 6 and either groups 7 or 8 at each time point except day 6. Differences between groups 7 and 8 were not significant at any time point.
4. Discussion
Our results with the recombinant subunit West Nile vaccine are very encouraging. After two subcutaneous immunizations with any of the recombinant antigen formulations containing 80E, the hamsters developed relatively high antibody titers to WNV, as determined by HI, CF and PRNT assays, which remained high for at least 6 months post booster vaccination. The PRNT titers were stable for at least 12 months post booster vaccination, but the HI and CF titers declined. The reason for this difference between the longevity of the PRNT antibody response and that of the HI and CF antibody responses is not clear. However, one possible explanation may be that these assays measure different antibody isotypes. In mice, viral neutralizing (PRNT) antibodies for dengue virus have been shown to reside primarily in the IgG2a subclass [8]. HI and CF antibodies may involve other isotypes, possibly IgM, which may decline much faster than the PRNT antibody isotype.
When challenged with live virus, the 80E vaccinated animals survived and remained disease-free when the challenge occurred up to 12 months post booster vaccination. Viremia was undetectable in these vaccinated animals when challenged up to 6 months post booster but was observed at very low levels when the challenge occurred 12 months post booster. In addition, animals immunized with NS1 without 80E were partially protected against challenge and developed lower levels of viremia which was cleared earlier than in adjuvant control animals similarly challenged. Although the experiments reported herein were conducted with vaccines formulated with only one adjuvant (ISCOMATRIX® adjuvant), in other similar experiments performed with vaccine formulations containing other adjuvants, e.g., a saponin plus a “CpG” oligonucleotide, complete protection was also observed in vaccinated hamsters (data not shown). In those and other experiments, the serum antibody titers among vaccinated hamsters were comparable to titers obtained in hamsters with a live attenuated chimeric West Nile virus vaccine candidate [6] and were only slightly less than titers found in hamsters surviving experimental infection with wild virus [4, 9]. In many of these experiments, there was an absence of a secondary antibody response, or a delayed response after challenge with the live virus, which supports the view that there was little virus replication in the vaccinated animals. Hamsters passively immunized with WNV immune serum and then challenged with WNV have a similar response [6].
One clear advantage of a recombinant subunit WNV vaccine over a live attenuated or chimeric vaccine is that no live virus is injected into the patient. This is of particular relevance for a WNV vaccine, since one of the presumed target groups for vaccination would include the elderly, immunosuppressed persons and other individuals with chronic disease conditions. Live virus vaccines are contraindicated in certain of these conditions because of the risk of adverse vaccine events [10–12].
Acknowledgments
This work was supported in part by contracts NO1-AI 25489 and NO1-AI30027, and grants 1 R43 AI052600-01A1 and 9 R44 NS52139-02A1, from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 2.Debrot MA, Lindsay LR, Barker IK, et al. West Nile virus surveillance and diagnosis: A Canadian perspective. Can J Infect Dis. 2003;14:105–114. doi: 10.1155/2003/575341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estrada-Franco JG, Navarro-Lopez R, Beasley DWC, et al. Isolation of West Nile virus in Mexico and serologic evidence of widespread circulation since July 2002. Emerg Infect Dis. 2003;9:1604–1607. doi: 10.3201/eid0912.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao SY, Guzman H, Zhang H, Travassos da Rosa APA, Tesh RB. West Nile virus infection in the golden hamster (Mesocricetus auratus): a model for West Nile encephalitis. Emerg Infect Dis. 2001;7:714–721. doi: 10.3201/eid0704.010420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman M, Clements D, Ogata S, et al. Preparation and immunogenic properties of a recombinant West Nile subunit vaccine. Vaccine. doi: 10.1016/j.vaccine.2006.08.018. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesh RB, Arroyo J, Travassos da Rosa APA, Guzman H, Xiao SY, Monath TP. Efficacy of killed virus vaccine, live attenuated chimeric virus vaccine, and passive immunization for prevention of West Nile virus encephalitis in hamster model. Emerg Infect Dis. 2002;8:1392–1397. doi: 10.3201/eid0812.020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Schmidt NJ, Emmons RW, editors. Diagnostic procedures for viral, rickettsial and chlamydial infections. 6. Washington: American Public Health Association; 1989. pp. 797–856. [Google Scholar]
- 8.Smucny JJ, Kelly EP, Macarthy O, King AD. Murine immunoglobulin G subclass responses following immunization with live dengue virus or a recombinant dengue envelope protein. Am J Trop Med Hyg. 1995;53:432–437. doi: 10.4269/ajtmh.1995.53.432. [DOI] [PubMed] [Google Scholar]
- 9.Tesh RB, Travassos da Rosa APA, Guzman H, Araujo TP, Xiao SY. Immunization with heterologous flaviviruses protects against fatal West Nile encephalitis. Emerg Infect Dis. 2002;8:245–251. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljungman P. Vaccination in the immunocompromised host. In: Plotkin SA, Orenstein WA, editors. Vaccines. 4. Philadelphia: Saunders; 2004. pp. 155–168. [Google Scholar]
- 11.Barwick RS, Marfin AA, Cetron MS. Yellow fever vaccine-associated disease. In: Scheld WM, Murray BE, Hughes JM, editors. Emerging infections 6. Washington: ASM Press; 2003. [Google Scholar]
- 12.Centers for Disease Control and Prevention. Notice to readers: Supplemental recommendations on adverse events following smallpox vaccine in the pre-event vaccination program: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2003;52:282–284. [PubMed] [Google Scholar]


