Abstract
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV) is linked to KS, primary effusion lymphomas (PEL), and a subset of multicentric Castleman’s disease (MCD). Transcript mapping studies using PEL cell lines have allowed preliminary classification of viral gene expression into constitutive (class I) and inducible (class II/III) categories. To determine whether viral gene expression differs in vivo, we examined tissue sections of KSHV-infected disorders, using specific antibodies against proteins that are representative of the different expression classes of KSHV genes. ORF73/LANA appears to be a surrogate marker for KSHV infection because it is constitutively expressed in vitro and in vivo in all KSHV-infected cells. Expression of vIRF1, vIL6, and PF-8 proteins in the infected B cells of MCD lymph nodes reproduces the expression pattern observed in TPA-stimulated KSHV-infected B-cell lines. In contrast, the protein expression of the inducible viral genes that we tested in KS and PEL biopsies is restricted to PF-8 and vIL6, respectively. The tightly restricted expression of KSHV proteins in vivo differs from the dysregulated expression of inducible KSHV genes in vitro and suggests that viral gene expression in KSHV-infected cell lines does not accurately reflect what occurs in diseased tissues. These differences may be related to either cell-specific or immune restriction of viral replication.
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV) is a human γ-herpesvirus involved in the pathogenesis of KS, primary effusion lymphoma (PEL), and a subset of multicentric Castleman’s disease (MCD). 1-3 KSHV can be stably propagated in vitro in B-cell lines derived from PELs, but currently it is not readily transmissible at high titer. 4-8 Similar to other herpesviruses, KSHV infection can be characterized as lytic or latent. During lytic replication, virions are packaged and released from the cell. This process requires DNA synthesis together with expression of virion structural protein genes and is believed to result in cell death. Latent infection, however, is characterized by the persistence of the viral genome as a covalently closed circular episome with limited viral gene expression. 9,10 Latent KSHV in cells derived from PEL can be induced into lytic replication by chemical treatment with tetradecanoyl phorbol acetate (TPA) or butyrate. 10,11
PEL-derived cell lines stimulated with phorbol esters or butyrate reveal three distinct classes of KSHV messenger RNAs (mRNAs) corresponding to constitutive (type I), constitutive/inducible (type II), and lytic/inducible (type III) transcripts. 12 The expression of selected genes can be further modulated by treatment with cycloheximide, phosphonoacetic acid, or both. 13 Most of the viral cytokines and signal transduction genes that are unique to KSHV are type II or III, inducible genes. 12 These genes encode proteins that have been postulated to play a central role in the pathogenesis of KSHV-related diseases.
A major drawback to studies on viral gene expression in tissue culture cell lines is that expression patterns in such systems may not reflect viral gene expression in infected tissues in situ. For example, Epstein-Barr virus (EBV) demonstrates different programs of virus latency in different cell types. 14 It is currently unknown whether the pattern of gene transcription observed in vitro in PEL cell lines is present in different types of KSHV-infected cells in vivo. Recent immunohistochemistry studies have shown that KS spindle cell, mantle zone B cells in MCD lymph nodes and PEL cells express KSHV LANA, 15-17 whereas vIL-6 is detectable in only a subpopulation of KSHV-infected hematopoietic cells. 18,19 In this study we used a panel of antibodies directed against KSHV LANA (ORF73), 20 vIL6 (K2), 18 vIRF1 (ORFK9), 21 and processivity factor-8 (PF-8) (ORF59). 22 These proteins represent type I-constitutive (ORF73/LANA), type II-constitutive/inducible (ORFK9/vIRF1 and ORF K2/vIL6), and type III-lytic/inducible (ORF59/PF-8) genes of KSHV. 12 Our results demonstrate that some KSHV genes can become dysregulated in tissue culture and that tissue-specific patterns of expression are not represented by tissue culture studies. Furthermore, different KSHV-associated diseases show distinct patterns of viral gene expression. Therefore KSHV, like EBV, exhibits multiple programs for viral gene expression.
Materials and Methods
Cell Lines
BC-1, 4 BCP-1, 5 and BCBL-1 8 KSHV-infected cells were maintained at 37°C and 5% CO2 in 1640 RPMI (GIBCO/BRL, Grand Island, NY) supplemented with 10% to 20% fetal calf serum (GIBCO/BRL). Induction of viral gene expression was performed by treatment of cells with 20 ng/ml of TPA (Sigma Chemical Co., St. Louis, MO). Ramos (EBV−/KSHV−) and P3HRI (EBV+/KSHV−) cells (ATCC, Gaithersburg, MD) were used as KSHV-negative control cell lines. Cytospins, cell pellets, or both were harvested after 48 hours. Cytospins were air dried overnight, fixed in acetone for 4 minutes at room temperature, air dried for 30 minutes, and then either processed for immunohistochemistry or stored at −80°C until use. Cell pellets were fixed overnight in 4% paraformaldehyde in 0.1 mol/L phosphate-buffered saline (PBS; pH7.4) and embedded in paraffin.
Tissue Cases and Controls
Fifteen KS skin lesions, 10 lymph nodes from patients with MCD, and biopsies from four cases of PEL were investigated. KS biopsies included both classical (n = 7) and human immunodeficiency virus (HIV)-associated (n = 8) cases and were representative of patch (n = 5), plaque (n = 7), and nodular (n = 3) stages of the disease. All 10 MCD cases were of the plasma cell variant, six from HIV-seronegative and four from HIV-seropositive individuals. In contrast, all four PEL cases were acquired immune deficiency syndrome (AIDS)-related. Frozen material (required for PF-8 immunostaining) was available for 8 of 15 KS lesions and 7 of 10 MCD cases. Frozen material was available from one of the four PEL cases. Frozen and paraffin-embedded sections from normal tonsils (n = 5) and skin biopsies (n = 5) were used as controls.
Antibodies
The panel of antibodies against KSHV used in this study specifically recognizes viral proteins representative of different classes of transcripts involved in virus replication (see Table 1 ▶ ). When applied to KSHV-infected cells, monoclonal antibodies and sera directed against different portions of the same KSHV protein and/or raised in different species showed the same staining pattern and labeled the same percentage of cells, with a range of variability of less than 5% (data not shown). Immunohistochemical procedures to reveal KSHV expression on paraffin-embedded biopsies were optimized by using sections from formalin-fixed/paraffin-embedded pellets of KSHV-infected PEL-derived (BC-1, BCP-1, BCBL-1) cell lines and uninfected control cells (RAMOS). The results on paraffin-embedded sections were compared with the results obtained on acetone-fixed cytospins of the same cell lines (data not shown). For a given antibody, the conditions were considered satisfactory when, in repeated experiments, the number of immunostained cells was the same on paraffin-embedded sections and cytospins, with a range of variability of less than 5% (data not shown). To obtain the same percentage of stained cells on the paraffin-embedded sections and on the cytospins, antibodies against LANA and vIRF1 required a microwave-ethylenediaminetetraacetic acid (EDTA) pretreatment of paraffin sections, 23 whereas the reactivity of antibodies against vIL6 did not require this treatment. The monoclonal antibody M11D1 against KSHV PF-8 was the only reagent that was nonreactive on paraffin sections and required frozen materials (see Table 1 ▶ ).
Table 1.
Antibodies against KSHV Proteins
| Antibody | Antigen/Source (reference) | KSHV gene/protein | Transcription class | Immuno- histochemistry* |
|---|---|---|---|---|
| R UK163 (rabbit) | BCBL-1 cells (20) | ORF73/LANA | Class I—constitutive | F/P(MW) |
| R 535 (rabbit) | GST-conj rec. protein (21) | ORFK9/v-IRF1 | Class II—constitutive/inducible | F/P(MW) |
| R 537 (rabbit) | GST-conj rec. protein | |||
| S 544 (sheep) | GST-conj rec. protein | |||
| R 394 (rabbit) | synthetic peptide, AA 218-432 (18) | ORFK2/v-IL6 | Class II—constitutive/inducible | F/P |
| S 546 (sheep) | GST-conj rec. protein | |||
| M 11D1 (mouse monoclonal) | TPA-induced BCBL-1 (22) | ORF59/PF-8 | Class III—lytic/inducible | F |
*F, reactive on cytospins and frozen sections; P, reactive on paraffin sections; MW, microwave treatment required; GST-conj rec., GST-conjugated recombinant.
Monoclonal antibodies or rabbit antisera against CD3, CD4, CD8, CD45, CD20, CD23, CD5, CD79, CD30, EMA, Ki67, CD34, vWF:Fact.VIII (DAKO, Glostrupp, Denmark), and CD138 (VIth Workshop on Human leukocyte Antigens, Osaka, Japan) were used to detect specific lymphocyte subpopulations as well as spindle cells in KS lesions.
Immunohistochemistry
Antibody binding was revealed using peroxidase-labeled goat anti-mouse or goat anti-rabbit antisera (DAKO) followed by tyramide amplification (DuPont/NEN, Boston, MA). Reactions were developed using diaminobenzidine (DAB; Sigma) or amino ethyl carbazole (AEC; DAKO) as chromogenic substrates, and sections were counterstained with hematoxylin. For double immunostaining, the second primary antibody was revealed using alkaline phosphatase-conjugated rabbit anti-mouse or swine anti-rabbit antisera and Fast Blue (Sigma) as chromogen. For immunofluorescence, fluorescein-isothiocyanate (FITC)-conjugated goat anti-mouse or goat anti-rabbit antisera (Southern Biotechnology, Birmingham, AL) were used alone or, for double staining, in combination with biotinylated horse anti-mouse (Vector Laboratories, Burlingame, CA) or goat anti-rabbit antisera (Southern Biotechnology) followed by Avidin Texas Red (Vector).
Results
Protein Expression in PEL-Derived BC-1, BCP-1, and BCBL-1 Cell Lines
Protein expression patterns were examined in three cell lines (BC-1, BCP-1, and BCBL-1) before and after TPA treatment (summarized in Table 2 ▶ ). Although LANA was expressed in all cells (Figure 1A) ▶ regardless of TPA treatment and appears to be a marker for KSHV infection, examination of the immunohistochemical profile for the remaining proteins reveals a tightly regulated expression pattern. The BC-1 cell line had constitutive low-level expression of vIRF1 and vIL6 (class II genes) but lacked expression of PF-8 (class III). In contrast, low-level PF-8 protein expression was present in BCBL-1 and BCP-1 cells without TPA treatment, which is consistent with these cell lines being producer cell lines with a low percentage of cells spontaneously entering into full lytic replication. The percentages of cells expressing vIRF1 and PF-8 increased approximately 20-fold after TPA treatment for all three cell lines, whereas vIL6, which was expressed at higher constitutive levels, increased only 3- to 5-fold (Table 3) ▶ . We found that the vIL6 staining pattern in KSHV-infected cell lines was less than initially reported, 18 probably due to technical artifacts in the earlier study that were related to antibody dilution and development. In TPA-stimulated BCBL-1 cells, double-staining studies demonstrated that more than 80% of cells expressing vIRF1 coexpressed PF-8, as compared with 10% of PF-8-positive cells expressing vIL6 (Table 4) ▶ . All antibodies against KSHV proteins were nonreactive by immunohistochemistry to RAMOS and P3HRI cells, as well as KSHV-negative tissue controls.
Table 2.
Expression of KSHV Proteins in Infected PEL-Derived Cell Lines versus KS, PEL, and mCD Tissue Samples
| KSHV protein | PEL-derived cell lines* | KS | MCD | PEL |
|---|---|---|---|---|
| LANA | + | + | + | + |
| v-IRF1 | + | − | + | − |
| v-IL6 | + | − | + | + |
| PF-8 | + | + | + | − |
*BCBL-1, BCP-1, BC-1.
Figure 1.
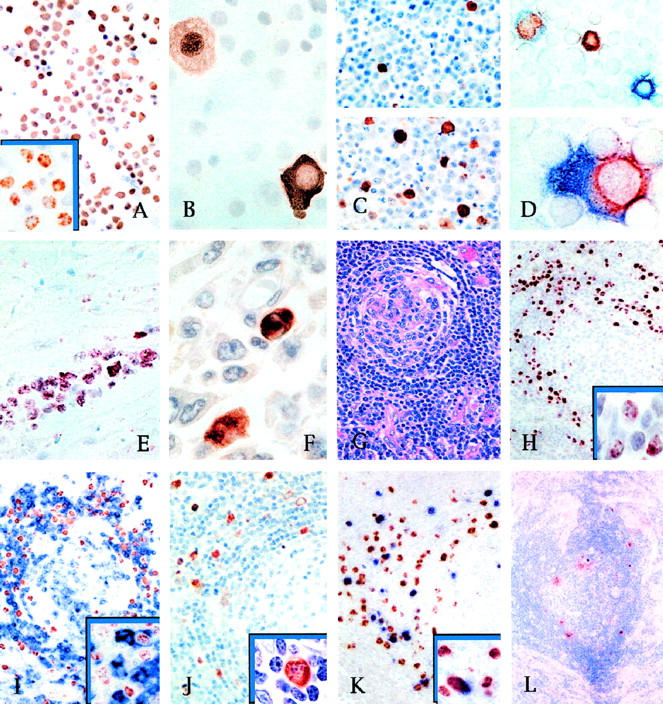
A: LANA is constitutively expressed in nearly all BCBL-1 cells as a speckled nuclear pattern (inset). Similar expression is seen also in BC-1 and BCP-1 cells (DAB/hematoxylin counterstain). B: vIRF1 in BCBL-1 cells is localized to the cytoplasm in one cell (right) and in the nucleus of another (left), consistent with nuclear translocation. C: Only 1% of BCBL-1 cells express vIRF1 without TPA (upper panel) but up to >20% (lower panel) after 48 hours of TPA stimulation (DAB/hematoxylin counterstain). D: With TPA stimulation (upper panel) BCBL-1 cells may express nuclear PF-8 alone (brown), cytoplasmic vIRF1 alone (blue), or may coexpress both proteins. In some cells double stained for PF-8 and vIRF1, the latter shows a pseudolinear intracytoplasmic pattern reminiscent of rough endoplasmic reticulum positivity (lower panel) (AEC/FastBlue; no counterstain). E: Section of myocardium with infiltrating PEL. All neoplastic cells express nuclear LANA (AEC/hematoxylin counterstain). F: Peritoneal biopsy with infiltrating PEL. Only a few tumor cells are immunopositive for vIL6 (DAB/hematoxylin counterstain). G: Hyalinization of germinal centers, targetoid mantle zone, and increased vascularity in a lymph node biopsy from plasma cell variant, KSHV-related multicentric Castleman’s disease (H&E stain). H: KSHV LANA protein expression is restricted to a subpopulation of mantle zone lymphocytes that show a speckled nuclear staining pattern (inset) (DAB/hematoxylin counterstain). I: Double-stained section of an MCD lymph node showing that nearly all of the KSHV-infected mantle zone lymphocytes expressing LANA (light brown) are negative for CD79 (blue), with only a few double-positive cells (inset). Similar findings are obtained by double staining with other B-cell antigens expressed by normal mantle zone lymphocytes. (DAB/FastBlue; no counterstain). J: MCD lymph node section with expression of vIL6 (brown) restricted to a subpopulation of mantle zone cells having immunoblastic morphology (inset) (DAB/hematoxylin counterstain). K: In double-stained sections, vIRF1 (blue) in mantle zone cells is also positive for LANA (inset). Double-stained lymphocytes, however, account for only 10% to 30% of the LANA-positive cells (DAB/FastBlue; no counterstain). L: MCD frozen section, same case as in C, shows that only rare cells at the border between mantle zone and germinal center express PF-8 (DAB/hematoxylin counterstain).
Table 3.
KSHV Protein Expression in Infected Cell Lines with and without TPA Stimulation
| KSHV protein | Staining pattern | % BCBL-1 | % BC-1 | % BCP-1 | |||
|---|---|---|---|---|---|---|---|
| TPA− | TPA+ | TPA− | TPA+ | TPA− | TPA+ | ||
| ORF73 | Nuclear, speckled | 100 | 100 | 100 | 100 | 100 | 100 |
| v-IRF1 | Nuclear and cytoplasmic, diffuse | 1 | 24 | <1 | 20 | 7 | 25 |
| v-IL6 | Cytoplasmic, diffuse | <1 | 2 | <1 | 6 | 4 | 15 |
| PF-8 | Nuclear, diffuse or membrane | 1 | 20 | ∼0 | 25 | ∼0 | 26 |
Table 4.
Coexpression of Inducible/Lytic KSHV Proteins in BCBL1 Cells with and without TPA Treatment
| KSHV proteins | TPA− (%) | TPA+ (%) | Summary |
|---|---|---|---|
| PF-8/v-IL6 | 0.1 | 1.8 | 80% vIL6+ are PF-8+ |
| PF-8 alone | 2.3 | 18 | 10% PF-8+ are vIL6+ |
| v-IL6 alone | 0.1 | 0.5 | |
| PF-8/v-IRF1 | 0.7 | 18 | 90% vIRF+ are PF-8+ |
| PF-8 alone | 0.2 | 3.8 | 80% PF-8+ are vIRF+ |
| v-IRF1 alone | 0.1 | 2.5 |
Expression of Viral Proteins in KSHV-Related Diseases
Kaposi’s Sarcoma
KSHV protein expression is highly restricted in KS lesions. LANA is the only protein examined that was expressed in most KS tumor cells. In KS lesions, regardless of histological stage, the nuclei of spindle and endothelial KS cells were positive for LANA in a typical speckled immunoreactivity pattern (Figure 2B) ▶ . Intratumoral arterioles and extralesional normal tissues, including epidermis and skin adnexa, were LANA negative (Figure 2C) ▶ . All clinical stages of KS, including patch, plaque, and nodular lesions, were positive for LANA expression. In all KS lesions, most CD45+ infiltrating leukocytes were CD68+ monocytic cells, with only a few and irregularly distributed CD3+ T or CD20+ B lymphocytes. Of these CD45+/CD68+ monocytes, less than 1% were LANA positive (Figure 2E) ▶ , and none expressed PF-8 or other KSHV proteins. No expression of vIRF1 or vIL6 was seen in any of the KS lesions. In four of five nodular lesions for which frozen material was available, the nuclei of rare (less than 1%) tumor spindle and endothelial cells were positive for PF-8 (Figure 2F) ▶ .
Figure 2.
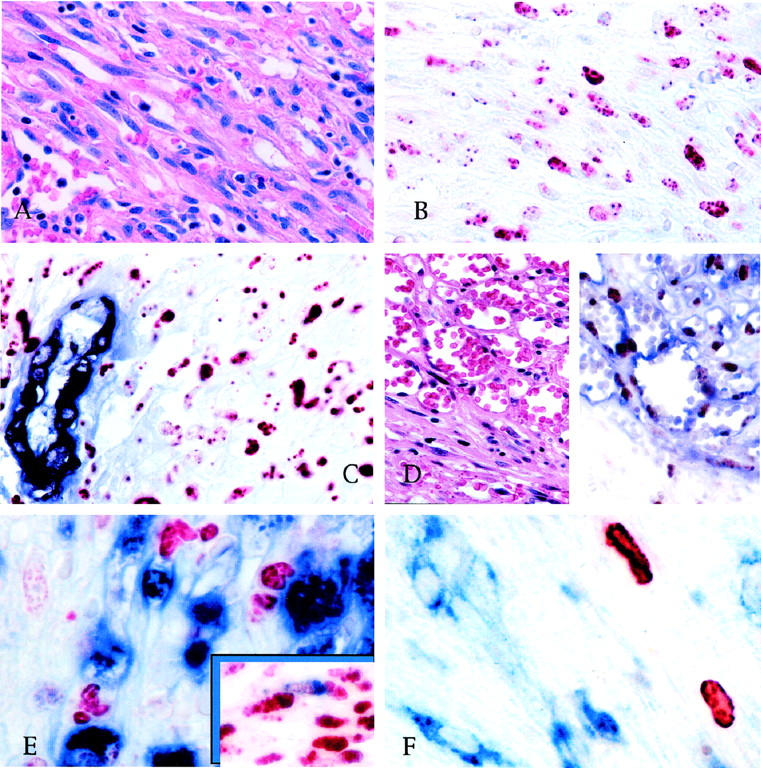
A: Spindle cell area with slit-like vascular spaces in nodular KS (H&E). B: Serial section of the case in A, stained for LANA, showing a speckled/granular nuclear reactivity in KS spindle cells (AEC/hematoxylin counterstain). C: In double-stained sections, LANA is detectable in the nuclei of KS cells (red) but not in the endothelial cells nor in the actin-positive smooth muscle cells (blue) of normal vessels entrapped within the tumor (AEC/FastBlue; no counterstain). D: Angiomatous area of a patch KS lesion containing CD34-positive endothelial cells (blue) lining KS vascular spaces, which also express LANA (brown) (H&E, left panel; DAB/FastBlue without counterstain, right panel). E: In double-stained sections, none of the KS cells expressing LANA are positive for CD68 (blue) or other leukocytic antigens, but rare LANA-positive KS spindle cells are positive for CD68 (inset). (DAB/FastBlue; no counterstain). F: Only rare cells are positive in KS frozen section stained for PF-8; CD68-positive monocytes (blue) are consistently negative (DAB/FastBlue; no counterstain).
PEL
Comparison of tumor biopsies to unstimulated cell lines shows several important differences between PEL cells growing in vivo and in vitro. As in KS lesions and PEL cell lines, LANA has a speckled nuclear pattern in virtually all PEL biopsy cells (Figure 1E) ▶ . In contrast, less than 5% of the cells were positive for vIL6 (Figure 1F) ▶ , and no immunostaining for vIRF1 protein was detected. Because vIRF1 can be detected at low levels in resting (ie, unstimulated) PEL cell lines, these data suggest that gene dysregulation has occurred in adaptation of the cells to culture.
MCD
In contrast to KS and PEL lesions, a broad pattern of KSHV protein expression is present in MCD tissues. Expression of KSHV proteins is confined to the follicular mantle zone, in which 10% to 30% of cells are positive for LANA (Figure 1H) ▶ . Double staining demonstrates that a subpopulation of these cells expresses vIL6 and vIRF1. The number of cells expressing these latter viral proteins, however, was highly variable among different follicles of the same lymph node, ranging from 5% to 25% of the LANA-positive cells (Figure 1, J and K) ▶ . In four cases for which frozen material was available, rare LANA-positive cells of the mantle zone were also positive for PF-8 (Figure 1L) ▶ . Outside the follicles, cells expressing KSHV proteins were an inconspicuous and occasional finding. When present, they were within poorly defined aggregates of B-lymphocytes in the paracortical areas or as single isolated cells in the marginal sinus. In all MCD cases, cells expressing KSHV proteins had large, irregular or round nuclei with marginated chromatin and medium-sized nucleoli (Figure 1J ▶ , inset). Cells expressing LANA were only occasionally positive for CD20 or CD79 (Figure 1I) ▶ but always negative for CD23 or CD5 or for the T-cell antigen CD3. In all but one MCD case, in which a single double-stained cell was observed, CD68+ monocytes were also negative.
Immunohistochemical results on KS, PEL, and CD biopsies are summarized in Table 2 ▶ . Extralesional normal tissues in the biopsies used in this study were consistently negative with all antibodies against KSHV proteins tested.
Discussion
The pattern of viral gene expression observed in vitro in PEL-derived cells is reproduced in vivo in MCD but not in KS nor in PEL. In MCD lymph nodes, cells expressing LANA are confined to the mantle zone of the lymphoid follicles. 24 These KSHV-infected cells are negative for T-cell or monocytic markers and are indistinguishable by morphology from normal mantle zone lymphocytes, although only a minority express CD20 or CD79 B-cell markers. A subset of the LANA-positive mantle zone cells, most of which have an immunoblastic morphology, expresses vIL6 as well as vIRF1 and, to lesser extent, PF-8. KSHV-infected mantle zone cells therefore reproduce the pattern of viral gene expression observed in TPA-stimulated PEL-derived cell lines, in which a subset of cells expresses class II and III genes. 6,8 As we reported previously 19 and as was subsequently confirmed by Staskus et al, 25 expression of vIL6 appears to be restricted to hematopoietic cells and, together with other inducible KSHV proteins, may be responsible for the hyperplastic changes typically observed in MCD lymph nodes.
Of the proteins assayed in this study, we found that expression is restricted to vIL6 alone in PEL tissue biopsies. In all 4 cases studied, vIL6 was detectable in less than 5% of the LANA-positive neoplastic cells, whereas vIRF1 and PF-8 proteins were not expressed. A difference in viral gene expression patterns between tissue culture cells and the parental tumor has been documented in EBV infection, in which cell lines derived from Burkitt’s lymphomas expressing type 1 latency in vivo acquire type 2 or type 3 latency in vitro (see 14 for review). Our findings suggest that KSHV protein expression may be different in vitro and in vivo. Therefore caution is needed in extending cell culture gene expression studies to patient tissues. Our findings also suggest that in vivo and in vitro expression of vIL6 can occur independently from the activation of the lytic replicative cascade.
In KS lesions, only LANA is expressed by spindle and endothelial KS cells, 16,24 with a small minority of the KS cell population expressing PF-8. This is consistent with in situ hybridization studies using T0.7 and T1.1 riboprobes as surrogate markers for latent and lytic virus replication, respectively. 25-27 However, in PEL tissue culture, both T0.7 and T1.1 are expressed constitutively, are inducible with TPA treatment, and are therefore defined as class II, constitutive/inducible. 12 Neither vIL6 nor vIRF1 was found to be expressed in endothelial or spindle KS cells, including the rare PF-8-positive cells. It has been suggested that primary KSHV infection of spindle cells does not play a central role in the initiation of the KS tumor; instead, infiltrating KSHV-infected mononuclear cells may be secondarily recruited to nascent uninfected KS tumors. 28 Our findings suggest otherwise, because infiltrating monocytes and lymphocytes in KS lesions were consistently negative for the KSHV proteins for which we assayed.
Immunohistochemical techniques address critical aspects of virus behavior in infected tissue culture cells and pathological lesions that cannot be explored by mRNA expression studies. Although extensive mRNA mapping of viral gene expression has been performed in KSHV-infected cell lines, this is the first report to compare the protein expression of a panel of constitutive and inducible genes in KSHV-associated disorders. Our study shows that KS, PEL, and MCD are characterized by differing patterns of KSHV protein expression. Currently, it is unclear whether these differences reflect the in vivo establishment of alternative types of latency, which enable KSHV to escape immunological surveillance, or whether these differences in patterns of expression are due to a cell/tissue-specific control of KSHV gene expression. These differences are disease specific and are likely to be relevant to understanding the pathogenesis of KSHV-infected diseases.
Acknowledgments
We thank Antoine Gessain (Institut Pasteur, Paris) for providing one of the PEL cases used in this study.
Footnotes
Address reprint requests to Yuan Chang, P&S 14–442, Dept. of Pathology, Columbia University, College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032. E-mail: yc70@columbia.edu.
Supported by National Institutes of Health grants CA67391, CA75911, and CA82056; II Programma Nazionale di Ricerca sull’AIDS1998-ISS Project Number 30.A.0.50; and the Lucy Pang Yoa Chang Foundation.
References
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS: Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994, 266:1865-1869 [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM: Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS- related body-cavity-based lymphomas. N Engl J Med 1995, 332:1186-1191 [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals HD, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al: Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 1995, 86:1276-1280 [PubMed] [Google Scholar]
- 4.Cesarman E, Moore PS, Rao RH, Inghirami G, Knowles DM, Chang Y: In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 1995, 86:2708-2714 [PubMed] [Google Scholar]
- 5.Boshoff C, Gao SJ, Healy LE, Matthews S, Thomas AJ, Coignet L, Warnke RA, Strauchen JA, Matutes E, Kamel OW, Moore PS, Weiss RA, Chang Y: Establishing a KSHV+ cell line (BCP-1) from peripheral blood and characterizing its growth in Nod/SCID mice. Blood 1998, 91:1671-1679 [PubMed] [Google Scholar]
- 6.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov VM, Grossberg S, Chang Y: Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol 1997, 71:314-324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renne R, Blackbourn D, Whitby D, Levy J, Ganem D: Limited transmission of Kaposi’s sarcoma-associated herpesvirus in cultured cells. J Virol 1998, 72:5182-5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D: Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med 1996, 2:342-346 [DOI] [PubMed] [Google Scholar]
- 9.Roizman B: The human herpesviruses. The family Herpesviridae. The Family Herpesviridae. Edited by B Roizman, RJ Whitley, C Lopez. New York, Raven Press, 1993, pp 1–9
- 10.Miller G, Rigsby MO, Heston L, Grogan E, Sun R, Metroka C, Levy JA, Gao SJ, Chang Y, Moore P: Antibodies to butyrate-inducible antigens of Kaposi’s sarcoma- associated herpesvirus in patients with HIV-1 infection. N Engl J Med 1996, 334:1292-1297 [DOI] [PubMed] [Google Scholar]
- 11.Offermann MK, Lin JC, Mar EC, Shaw R, Yang J, Medford RM: Antioxidant-sensitive regulation of inflammatory-response genes in Kaposi’s sarcoma cells. J Acquir Immune Defic Syndr Hum Retrovirol 1996, 13:1-11 [DOI] [PubMed] [Google Scholar]
- 12.Sarid R, Flore O, Bohenzky RA, Chang Y, Moore PS: Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J Virol 1998, 72:1005-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, Haase A, Miller G: Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol 1999, 73:2232-2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rickinson AB, Kieff E: Epstein-Barr virus. Fields Virology, Edited by BN Fields, DM Knipe, PM Howley. Philadelphia: Lippincott-Raven, 1956, pp. 2397–2446
- 15.Kedes DH, Lagunoff M, Renne R, Ganem D: Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J Clin Invest 1997, 100:2606-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellam P, Bourboulia D, Dupin N, Shotton C, Fisher C, Talbot S, Boshoff C, Weiss RA: Characterization of monoclonal antibodies raised against the latent nuclear antigen of human herpesvirus 8. J Virol 1999, 73:5149-5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainbow L, Platt GM, Simpson GR, Sarid R, Gao SJ, Stoiber H, Herrington CS, Moore PS, Schulz TF: The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol 1997, 71:5915-5921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore PS, Boshoff C, Weiss RA, Chang Y: Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 1996, 274:1739-1744 [DOI] [PubMed] [Google Scholar]
- 19.Parravicini C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore PS, Chang Y: Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman’s disease. Am J Pathol 1997, 151:1517-1522 [PMC free article] [PubMed] [Google Scholar]
- 20.Moses AV, Fish KN, Ruhl R, Smith PP, Strussenberg JG, Zhu L, Chandran B, N JA: Long-term infection and transformation of dermal microvascular endothelial cells by human herpesvirus 8. J Virol 1999, 73:6892–6902 [DOI] [PMC free article] [PubMed]
- 21.Jayachandra S, Low KG, Thlick A, Yu J, Ling PD, Chang Y, Moore PS: Three unrelated viral transforming proteins (vIRF, EBNA2 and E1A) induce the MYC oncogene through the interferon-responsive PRF element using different transcription coadaptors. Proc Natl Acad Sci USA 1999, 96:11566-11571 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Chan SR, Bloomer C, Chandran B: Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology 1998, 240:118-126 [DOI] [PubMed] [Google Scholar]
- 23.Cattoretti G, Pileri S, Parravicini C, Becker M, Poggi S, Bifulco C, Key G, D’Amato L, Sabattini E, Feudale E, Rilke F: Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol 1993, 171:83-98 [DOI] [PubMed] [Google Scholar]
- 24.Dupin N, Fisher C, Kellam P, Ariad S, Tulliez M, Franck N, van ME, Salmon D, Gorin I, Escande JP, Weiss RA, Alitalo K, Boshoff C: Distribution of human herpesvirus-8 latently infected cells in Kaposi’s sarcoma, multicentric Castleman’s disease, and primary effusion lymphoma. Proc Natl Acad Sci USA 1999, 96:4546–4551 [DOI] [PMC free article] [PubMed]
- 25.Staskus KA, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett SH, Haase AT: Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi’s sarcoma, primary effusion lymphoma, and multicentric Castleman’s disease. J Virol 1999, 73:4181-4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturzl M, Blasig C, Schreier A, Neipel F, Hohenadl C, Cornali E, Ascherl G, Esser S, Brockmeyer NH, Ekman M, Kaaya EE, Tschachler E, Biberfeld P: Expression of HHV-8 latency-associated T0.7 RNA in spindle cells and endothelial cells of AIDS-associated, classical and African Kaposi’s sarcoma. Int J Cancer 1997, 72:68–71 [DOI] [PubMed]
- 27.Staskus KA, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson DJ, Ganem D, Haase AT: Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol 1997, 71:715-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer NH, Tschachler E, Colombini S, Ensoli B, Sturzl M: Monocytes in Kaposi’s sarcoma lesions are productively infected by human herpesvirus 8. J Virol 1997, 71:7963-7968 [DOI] [PMC free article] [PubMed] [Google Scholar]


