Abstract
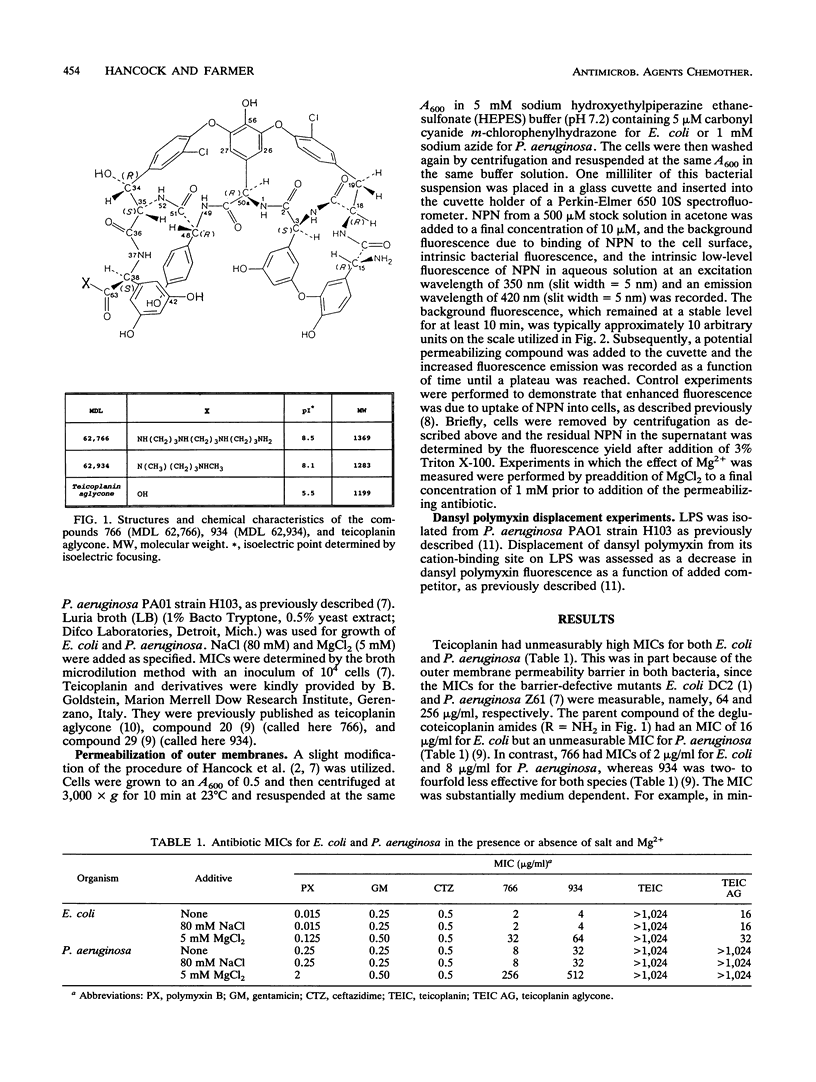
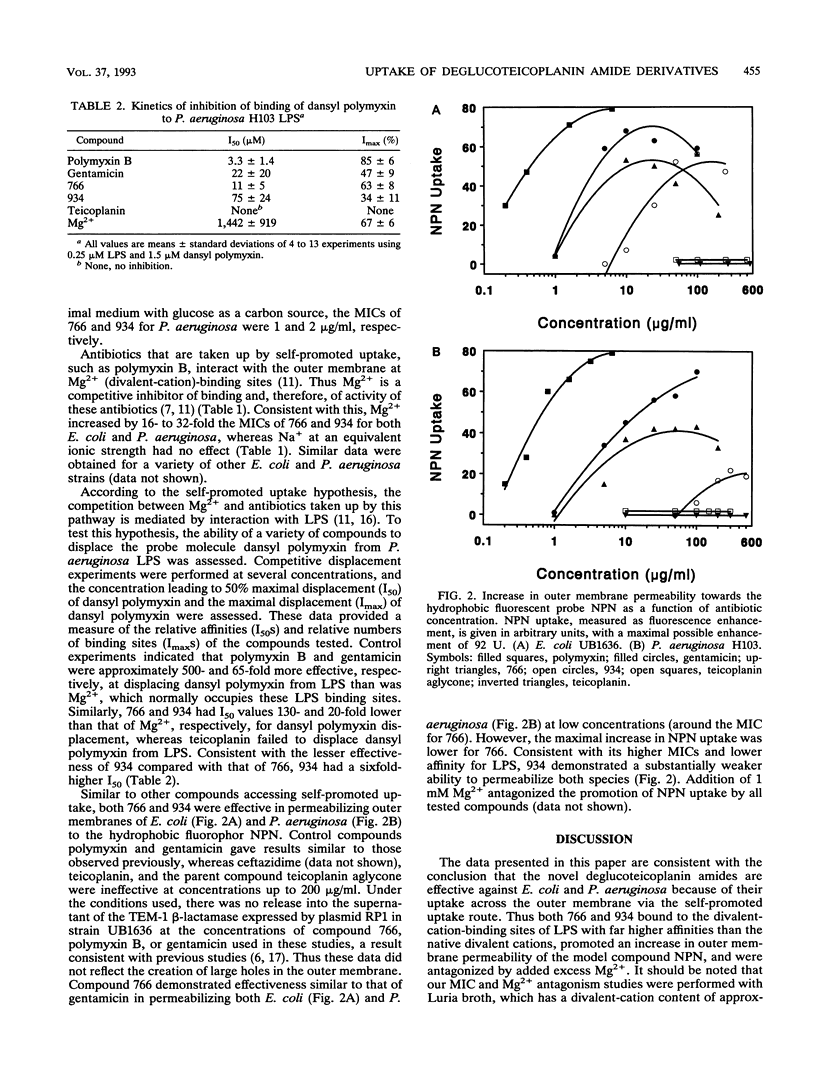
Teicoplanin is a glycopeptide antibiotic which is ineffective against gram-negative bacteria because of its inability to penetrate the outer membrane. Removal of the sugar residues and attachment of polyamines to carbon 63 yielded two dibasic deglucoteicoplanin amides, MDL 62,766 (766) and MDL 62,934 (934), with moderate MICs for Escherichia coli (2 to 4 micrograms/ml) and Pseudomonas aeruginosa (8 to 32 micrograms/ml) compared with those of the monobasic teicoplanin aglycone (16 and > 1,024 micrograms/ml, respectively). MICs were increased 16- to 32-fold by Mg2+ supplementation of Luria broth but not by Na+ supplementation at an equivalent ionic strength. Both 766 and 934 were capable of binding to P. aeruginosa lipopolysaccharide (LPS) at Mg(2+)-binding sites, as assessed by dansyl polymyxin displacement experiments. Furthermore, both compounds increased E. coli and P. aeruginosa outer membrane permeability to the hydrophobic fluorescent probe 1-N-phenylnaphthylamine (NPN), whereas the parent compounds teicoplanin aglycone and teicoplanin and the beta-lactam ceftazidime were totally ineffective. Addition of 1 mM Mg2+ blocked the increase in outer membrane permeability. Compound 766 had a lower MIC than 934 for both bacteria tested, bound to LPS with a higher affinity, and permeabilized outer membranes to NPN at lower concentrations. We propose that both deglucoteicoplanin amides exhibit increased gram-negative activity by virtue of their ability to access the self-promoted uptake pathway across the outer membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farmer S., Li Z. S., Hancock R. E. Influence of outer membrane mutations on susceptibility of Escherichia coli to the dibasic macrolide azithromycin. J Antimicrob Chemother. 1992 Jan;29(1):27–33. doi: 10.1093/jac/29.1.27. [DOI] [PubMed] [Google Scholar]
- Gilbert D. N., Kutscher E., Ireland P., Barnett J. A., Sanford J. P. Effect of the concentrations of magnesium and calcium on the in-vitro susceptibility of Pseudomonas aeruginosa to gentamicin. J Infect Dis. 1971 Dec;124 (Suppl):S37–S45. doi: 10.1093/infdis/124.supplement_1.s37. [DOI] [PubMed] [Google Scholar]
- Greenwood D. Microbiological properties of teicoplanin. J Antimicrob Chemother. 1988 Jan;21 (Suppl A):1–13. doi: 10.1093/jac/21.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):713–720. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Farmer S. W., Li Z. S., Poole K. Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of Escherichia coli. Antimicrob Agents Chemother. 1991 Jul;35(7):1309–1314. doi: 10.1128/aac.35.7.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Role of porins in outer membrane permeability. J Bacteriol. 1987 Mar;169(3):929–933. doi: 10.1128/jb.169.3.929-933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malabarba A., Ciabatti R., Kettenring J., Scotti R., Candiani G., Pallanza R., Berti M., Goldstein B. P. Synthesis and antibacterial activity of a series of basic amides of teicoplanin and deglucoteicoplanin with polyamines. J Med Chem. 1992 Oct 30;35(22):4054–4060. doi: 10.1021/jm00100a010. [DOI] [PubMed] [Google Scholar]
- Malabarba A., Trani A., Strazzolini P., Cietto G., Ferrari P., Tarzia G., Pallanza R., Berti M. Synthesis and biological properties of N63-carboxamides of teicoplanin antibiotics. Structure-activity relationships. J Med Chem. 1989 Nov;32(11):2450–2460. doi: 10.1021/jm00131a007. [DOI] [PubMed] [Google Scholar]
- Moore R. A., Bates N. C., Hancock R. E. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob Agents Chemother. 1986 Mar;29(3):496–500. doi: 10.1128/aac.29.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Schoenknecht F. D., Kenny M. A., Sherris J. C. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J Infect Dis. 1974 Nov;130(5):454–463. doi: 10.1093/infdis/130.5.454. [DOI] [PubMed] [Google Scholar]
- Rivera M., Hancock R. E., Sawyer J. G., Haug A., McGroarty E. J. Enhanced binding of polycationic antibiotics to lipopolysaccharide from an aminoglycoside-supersusceptible, tolA mutant strain of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1988 May;32(5):649–655. doi: 10.1128/aac.32.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer J. G., Martin N. L., Hancock R. E. Interaction of macrophage cationic proteins with the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1988 Mar;56(3):693–698. doi: 10.1128/iai.56.3.693-698.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Polycations as outer membrane-disorganizing agents. Antimicrob Agents Chemother. 1983 Jul;24(1):114–122. doi: 10.1128/aac.24.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T., Sarvas M. Decreased binding of polymyxin by polymyxin-resistant mutants of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):664–667. doi: 10.1128/jb.139.2.664-667.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


