Abstract
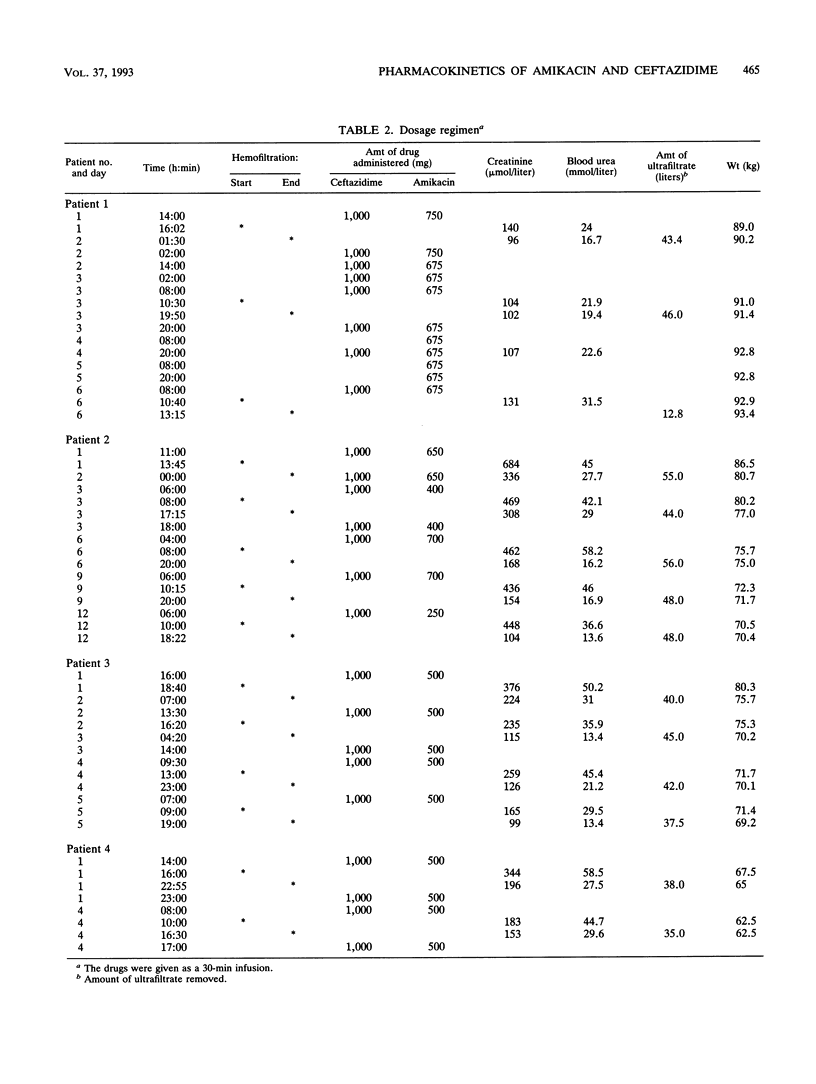
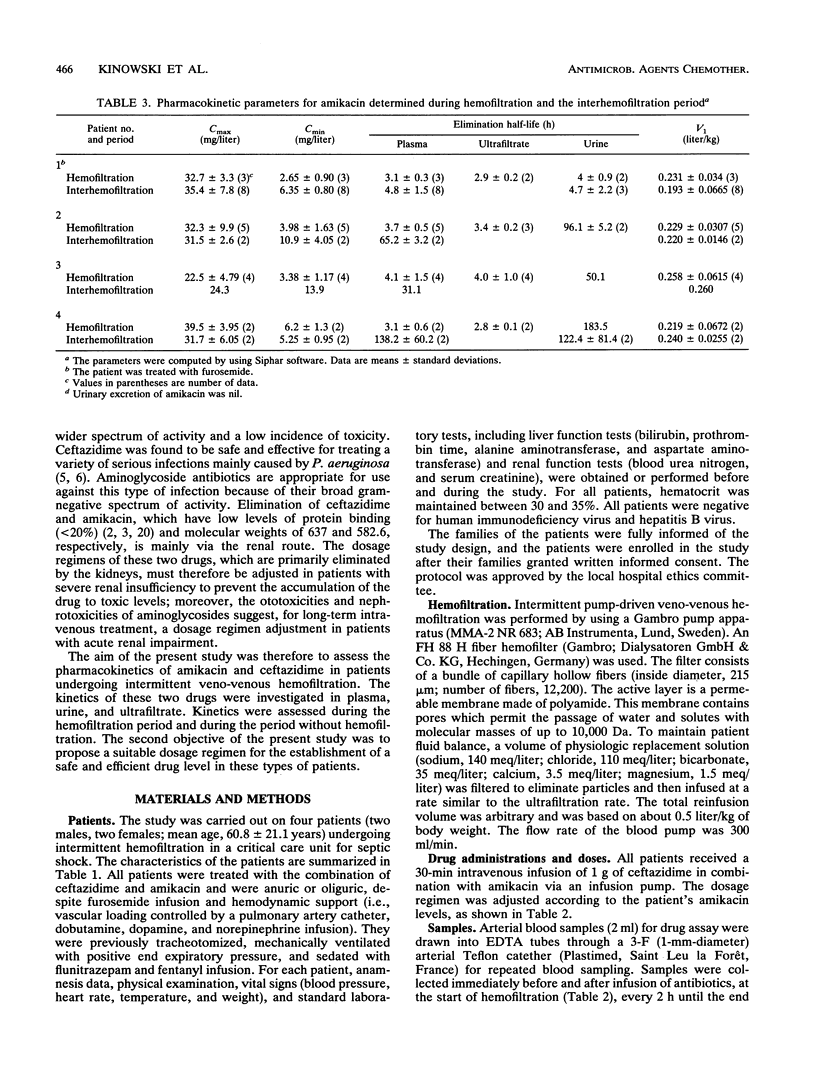
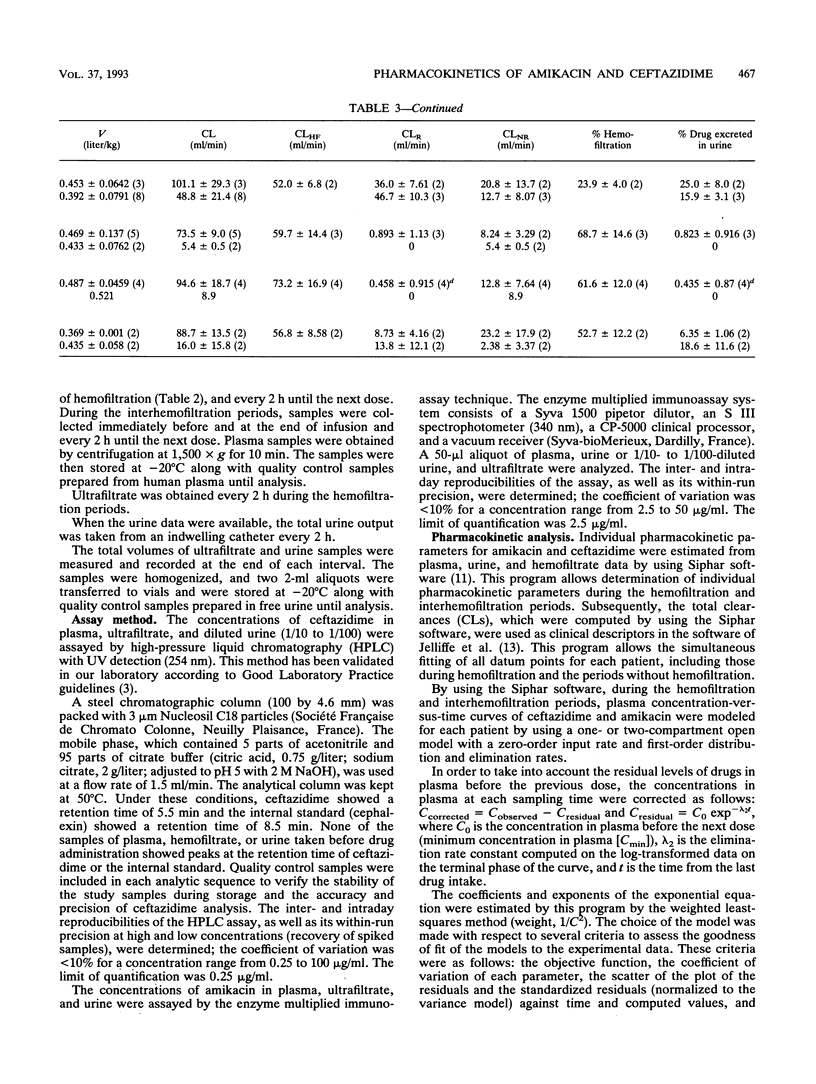
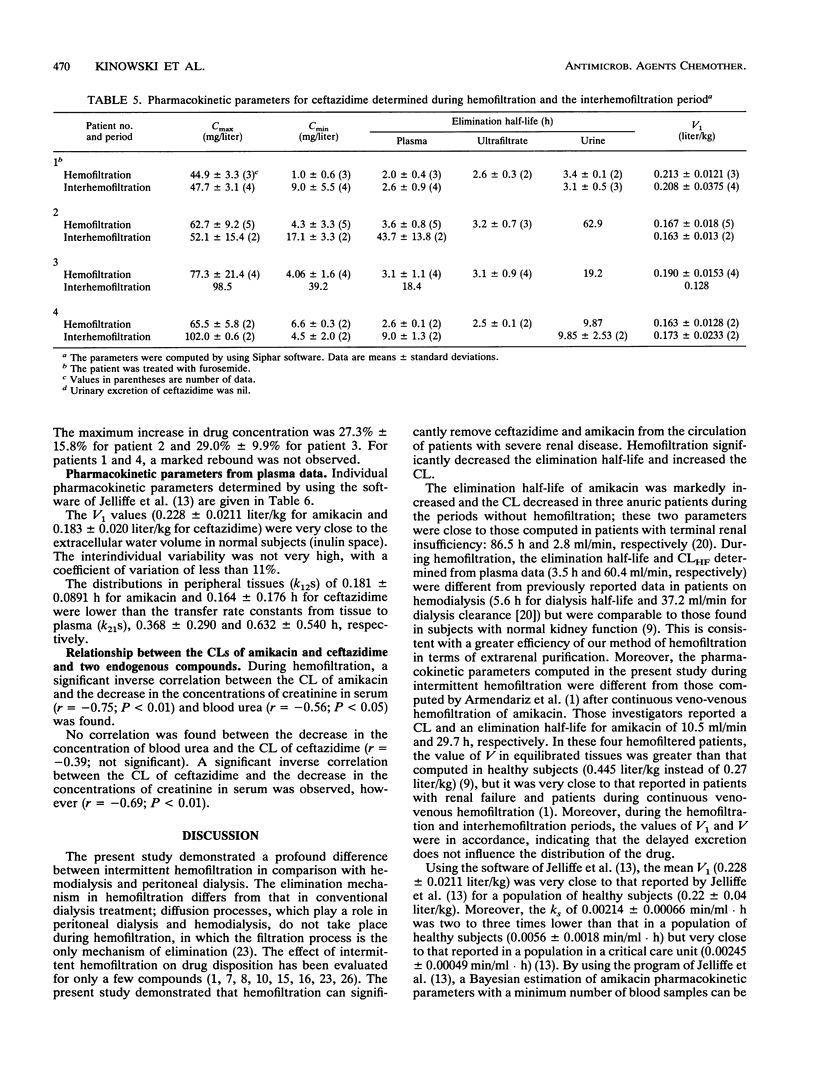
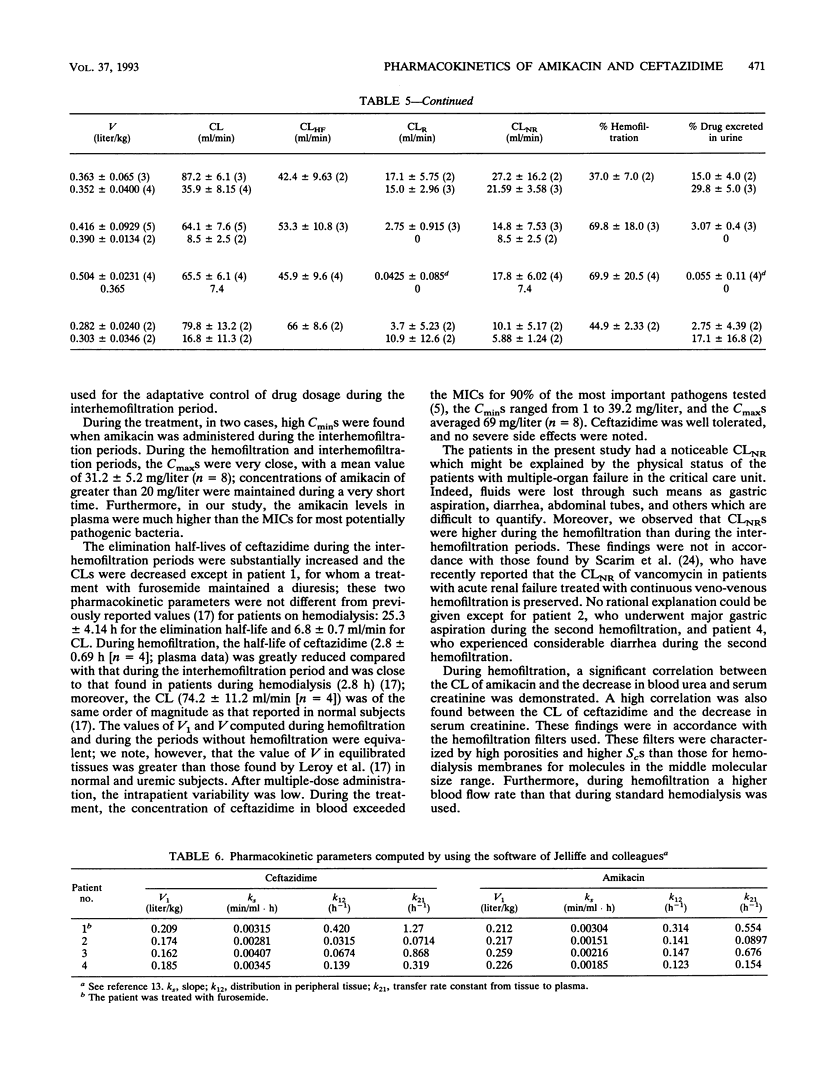
The pharmacokinetic parameters of amikacin and ceftazidime were assessed in four patients undergoing hemofiltration for septic shock. The parameters were assessed during hemofiltration and in the interim period. The concentration-time profiles of these two drugs in plasma, urine, and ultrafiltrate were investigated after intravenous perfusion (30 min). In all cases a 1-g dose of ceftazidime was administered; for amikacin, the dosage regimen was adjusted according to the patient's amikacin levels (250 to 750 mg). Concentrations of drug in all samples were assayed by high-performance liquid chromatography with UV detection for ceftazidime and by enzyme multiplied immunoassay for amikacin. The elimination half-life (t1/2) and the total clearance of amikacin ranged from 31.1 to 138.2 h and from 5.4 to 8.9 ml/min, respectively, during the interhemofiltration period in anuric patients. Hemofiltration substantially decreased the t1/2 (3.5 +/- 0.49 h) and increased the total clearance (89.5 +/- 11.8 ml/min). The hemofiltration clearance of amikacin represented 71% of the total clearance, and the hemofiltration process removed, on average, 60% of the dose. During hemofiltration, the elimination t1/2 of ceftazidime (2.8 +/- 0.69 h) was greatly reduced and the total clearance increased (74.2 +/- 11.2 ml/min) compared with those in the interhemofiltration period (9 to 43.7 h and 7.4 to 16.8 ml/min, respectively). About 55% of the administered dose was recovered in the filtrate, and the hemofiltration clearance of ceftazidime was 46 +/- 14.3 ml/min. A redistribution phenomenon (rebound) in the amikacin and ceftazidime concentrations in plasma (35 and 28%, respectively) was reported after hemofiltration in two patients. The MICs for 90% of the most important pathogens were exceeded by the concentrations of the two drugs in plasma during the whole treatment of these patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoni G., Arosio E., Raimondi M. G., Apolloni E., Passarella E., Lechi A., Velo G. P. Distribution of ceftazidime in ascitic fluid. Antimicrob Agents Chemother. 1984 Jun;25(6):760–763. doi: 10.1128/aac.25.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressolle F., de la Coussaye J. E., Ayoub R., Fabre D., Gomeni R., Saissi G., Eledjam J. J., Galtier M. Endotracheal and aerosol administrations of ceftazidime in patients with nosocomial pneumonia: pharmacokinetics and absolute bioavailability. Antimicrob Agents Chemother. 1992 Jul;36(7):1404–1411. doi: 10.1128/aac.36.7.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock V., Verbeelen D., Maes V., Sennesael J. Pharmacokinetics of vancomycin in patients undergoing haemodialysis and haemofiltration. Nephrol Dial Transplant. 1989;4(7):635–639. [PubMed] [Google Scholar]
- Garraffo R., Drugeon H. B., Dellamonica P., Bernard E., Lapalus P. Determination of optimal dosage regimen for amikacin in healthy volunteers by study of pharmacokinetics and bactericidal activity. Antimicrob Agents Chemother. 1990 Apr;34(4):614–621. doi: 10.1128/aac.34.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladziwa U., Krishna D. R., Klotz U., Ittel T. H., Schunkert H., Glöckner W. M., Mann H. Pharmacokinetics of ranitidine in patients undergoing haemofiltration. Eur J Clin Pharmacol. 1988;35(4):427–430. doi: 10.1007/BF00561377. [DOI] [PubMed] [Google Scholar]
- Gotloib L., Barzilay E., Shustak A., Waiss Z., Lev A. Hemofiltration in severe septic adult respiratory distress syndrome associated with varicella. Intensive Care Med. 1985;11(6):319–322. doi: 10.1007/BF00273545. [DOI] [PubMed] [Google Scholar]
- Keller F., Offermann G., Scholle J. Kinetics of the redistribution phenomenon after extracorporeal elimination. Int J Artif Organs. 1984 Jul;7(4):181–188. [PubMed] [Google Scholar]
- Kraft D., Lode H. Elimination of ampicillin and gentamicin by hemofiltration. Klin Wochenschr. 1979 Feb 15;57(4):195–196. doi: 10.1007/BF01477408. [DOI] [PubMed] [Google Scholar]
- Kramer P., Matthias C., Matthaei D., Scheler F. Elimination of cardiac glycosides through hemofiltration. J Dial. 1977;1(7):689–695. doi: 10.3109/08860227709037664. [DOI] [PubMed] [Google Scholar]
- Leroy A., Leguy F., Borsa F., Spencer G. R., Fillastre J. P., Humbert G. Pharmacokinetics of ceftazidime in normal and uremic subjects. Antimicrob Agents Chemother. 1984 May;25(5):638–642. doi: 10.1128/aac.25.5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke G. R., O'Connell M. B., Collins A. J., Keshaviah P. R. Disposition of vancomycin during hemofiltration. Clin Pharmacol Ther. 1986 Oct;40(4):425–430. doi: 10.1038/clpt.1986.201. [DOI] [PubMed] [Google Scholar]
- Regeur L., Colding H., Jensen H., Kampmann J. P. Pharmacokinetics of amikacin during hemodialysis and peritoneal dialysis. Antimicrob Agents Chemother. 1977 Feb;11(2):214–218. doi: 10.1128/aac.11.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renneberg J., Walder M. Postantibiotic effects of imipenem, norfloxacin, and amikacin in vitro and in vivo. Antimicrob Agents Chemother. 1989 Oct;33(10):1714–1720. doi: 10.1128/aac.33.10.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J. M., Walker J. F., Avila A., Petrakis A., Finley R. J., Sibbald W. J., Linton A. L. Renal and cardiovascular response to nonhypotensive sepsis in a large animal model with peritonitis. Surgery. 1985 Feb;97(2):205–214. [PubMed] [Google Scholar]
- Rumpf K. W., Rieger J., Doht B., Ansorg R., Scheler F. Drug elimination by hemofiltration. J Dial. 1977;1(7):677–678. doi: 10.3109/08860227709037663. [DOI] [PubMed] [Google Scholar]
- Weiss L. G., Cars O., Danielson B. G., Grahnen A., Wikström B. Pharmacokinetics of intravenous cefuroxime during intermittent and continuous arteriovenous hemofiltration. Clin Nephrol. 1988 Nov;30(5):282–286. [PubMed] [Google Scholar]
- Weiss L. G. Clinical aspects and applications of hemofiltration. Scand J Urol Nephrol Suppl. 1989;118:1–64. [PubMed] [Google Scholar]


