Abstract
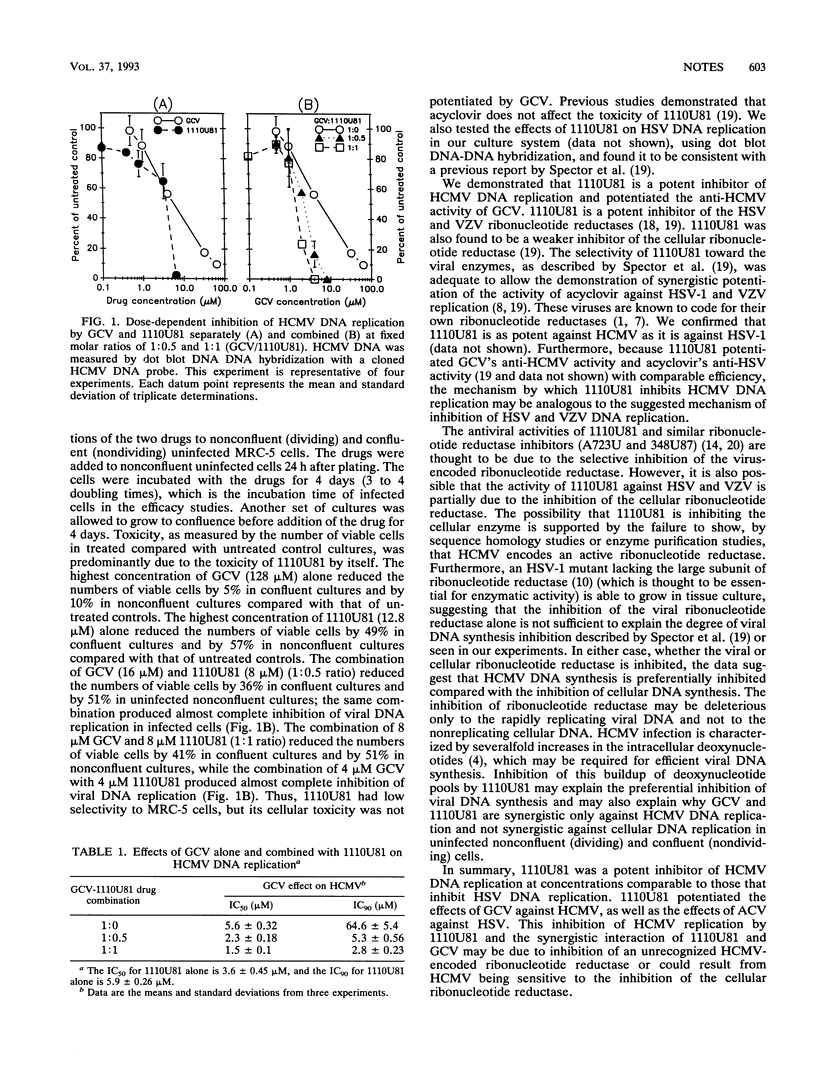
We studied the effects of 2-acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (1110U81 or A1110U), a potent inhibitor of the ribonucleotide reductases encoded by herpes simplex virus types 1 and 2 and by varicella-zoster virus, against human cytomegalovirus (HCMV) replication in infected MRC-5 cells. We show that 1110U81 is a potent inhibitor of HCMV DNA replication (50% inhibitory concentration [IC50], 3.6 microM; IC90, 5.6 microM) and also potentiates the effects of ganciclovir (GCV) against HCMV. The IC90 of GCV is reduced from 65 microM when GCV alone is given to 2.8 microM when GCV is combined with 1110U81 at a molar ratio of 1:1.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averett D. R., Furman P. A., Spector T. Ribonucleotide reductase of herpes simplex virus type 2 resembles that of herpes simplex virus type 1. J Virol. 1984 Dec;52(3):981–983. doi: 10.1128/jvi.52.3.981-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averett D. R., Lubbers C., Elion G. B., Spector T. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J Biol Chem. 1983 Aug 25;258(16):9831–9838. [PubMed] [Google Scholar]
- Berenbaum M. C. A method for testing for synergy with any number of agents. J Infect Dis. 1978 Feb;137(2):122–130. doi: 10.1093/infdis/137.2.122. [DOI] [PubMed] [Google Scholar]
- Biron K. K., Stanat S. C., Sorrell J. B., Fyfe J. A., Keller P. M., Lambe C. U., Nelson D. J. Metabolic activation of the nucleoside analog 9-[( 2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2473–2477. doi: 10.1073/pnas.82.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. C., Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- D'Aquila R. T., Hayward G. S., Summers W. C. Physical mapping of the human cytomegalovirus (HCMV) (Towne) DNA polymerase gene: DNA-mediated transfer of a genetic marker for an HCMV gene. Virology. 1989 Jul;171(1):312–316. doi: 10.1016/0042-6822(89)90546-1. [DOI] [PubMed] [Google Scholar]
- Dutia B. M. Ribonucleotide reductase induced by herpes simplex virus has a virus-specified constituent. J Gen Virol. 1983 Mar;64(Pt 3):513–521. doi: 10.1099/0022-1317-64-3-513. [DOI] [PubMed] [Google Scholar]
- Ellis M. N., Lobe D. C., Spector T. Synergistic therapy by acyclovir and A1110U for mice orofacially infected with herpes simplex viruses. Antimicrob Agents Chemother. 1989 Oct;33(10):1691–1696. doi: 10.1128/aac.33.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidts W. L., Ginsburg M., Pearson G. R. Neutralization of Epstein-Barr virus-induced ribonucleotide reductase with antibody to the major restricted early antigen polypeptide. Virology. 1989 May;170(1):330–333. doi: 10.1016/0042-6822(89)90390-5. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Weller S. K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988 Jan;62(1):196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S., Gibson W., Hayward G. S. Identification of the lytic origin of DNA replication in human cytomegalovirus by a novel approach utilizing ganciclovir-induced chain termination. J Virol. 1990 Dec;64(12):6184–6195. doi: 10.1128/jvi.64.12.6184-6195.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzeh F. M., Lietman P. S. Intranuclear accumulation of subgenomic noninfectious human cytomegalovirus DNA in infected cells in the presence of ganciclovir. Antimicrob Agents Chemother. 1991 Sep;35(9):1818–1823. doi: 10.1128/aac.35.9.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunting D., Henderson J. F. Models of the regulation of ribonucleotide reductase and their evaluation in intact mammalian cells. CRC Crit Rev Biochem. 1982;13(4):325–348. doi: 10.3109/10409238209108713. [DOI] [PubMed] [Google Scholar]
- Karlsson A., Harmenberg J. Effects of ribonucleotide reductase inhibition on pyrimidine deoxynucleotide metabolism in acyclovir-treated cells infected with herpes simplex virus type 1. Antimicrob Agents Chemother. 1988 Jul;32(7):1100–1102. doi: 10.1128/aac.32.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langelier Y., Buttin G. Characterization of ribonucleotide reductase induction in BHK-21/C13 Syrian hamster cell line upon infection by herpes simplex virus (HSV). J Gen Virol. 1981 Nov;57(Pt 1):21–31. doi: 10.1099/0022-1317-57-1-21. [DOI] [PubMed] [Google Scholar]
- Ponce de Leon M., Eisenberg R. J., Cohen G. H. Ribonucleotide reductase from herpes simplex virus (types 1 and 2) infected and uninfected KB cells: properties of the partially purified enzymes. J Gen Virol. 1977 Jul;36(1):163–173. doi: 10.1099/0022-1317-36-1-163. [DOI] [PubMed] [Google Scholar]
- Porter D. J., Harrington J. A., Spector T. Herpes simplex virus type 1 ribonucleotide reductase: selective and synergistic inactivation by A1110U and its iron complex. Biochem Pharmacol. 1990 Feb 15;39(4):639–646. doi: 10.1016/0006-2952(90)90140-g. [DOI] [PubMed] [Google Scholar]
- Spector T., Harrington J. A., Morrison R. W., Jr, Lambe C. U., Nelson D. J., Averett D. R., Biron K., Furman P. A. 2-Acetylpyridine 5-[(dimethylamino)thiocarbonyl]-thiocarbonohydrazone (A1110U), a potent inactivator of ribonucleotide reductases of herpes simplex and varicella-zoster viruses and a potentiator of acyclovir. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1051–1055. doi: 10.1073/pnas.86.3.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T., Harrington J. A., Porter D. J. Herpes and human ribonucleotide reductases. Inhibition by 2-acetylpyridine 5-[(2-chloroanilino)-thiocarbonyl]-thiocarbonohydrazone (348U87). Biochem Pharmacol. 1991 Jun 21;42(1):91–96. doi: 10.1016/0006-2952(91)90685-x. [DOI] [PubMed] [Google Scholar]
- Spector T., Lobe D. C., Ellis M. N., Blumenkopf T. A., Szczech G. M. Inactivators of herpes simplex virus ribonucleotide reductase: hematological profiles and in vivo potentiation of the antiviral activity of acyclovir. Antimicrob Agents Chemother. 1992 May;36(5):934–937. doi: 10.1128/aac.36.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Stinski M. F. Cloning of the human cytomegalovirus genome as endonuclease XbaI fragments. Gene. 1981 Dec;16(1-3):207–216. doi: 10.1016/0378-1119(81)90077-9. [DOI] [PubMed] [Google Scholar]


