Abstract
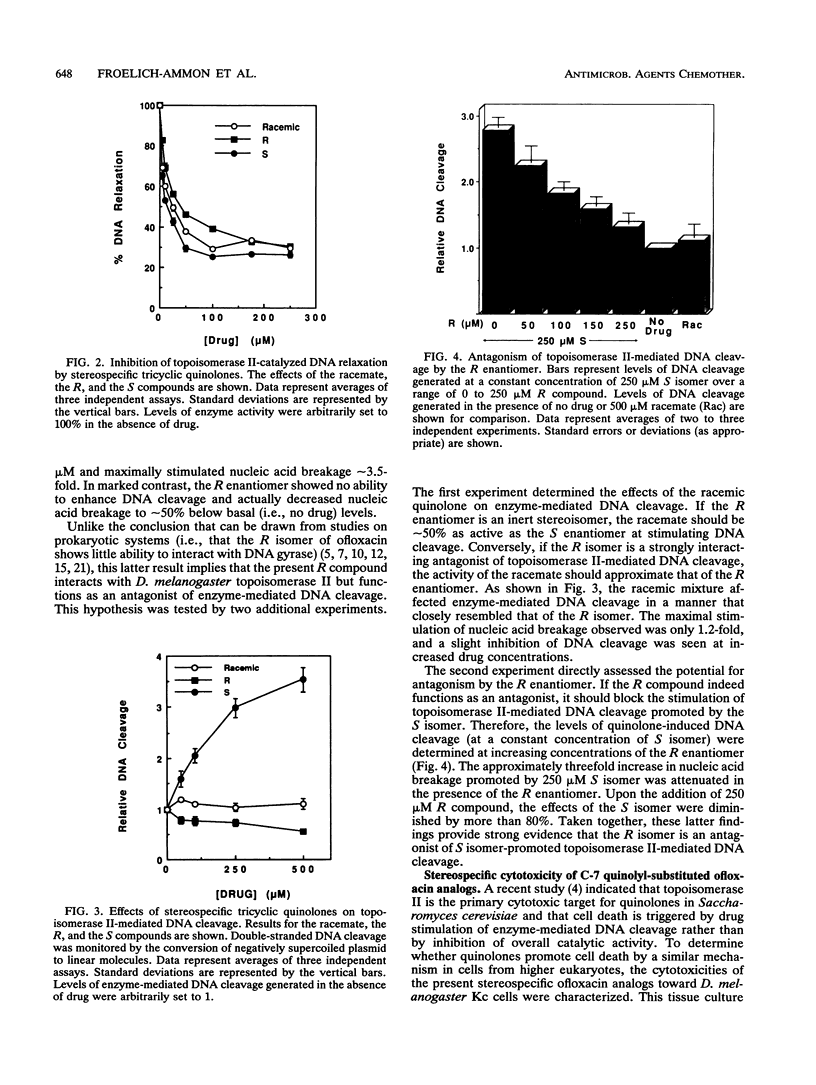
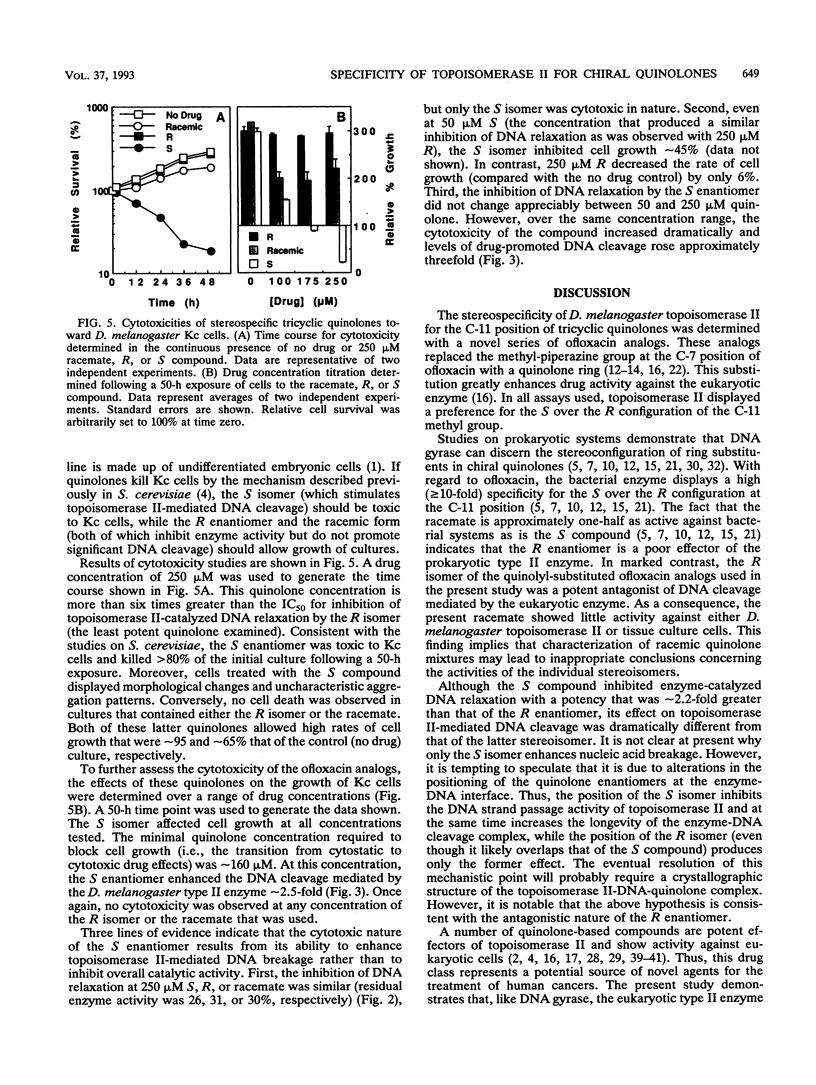
A series of novel C-7 quinolyl-substituted enantiomers of ofloxacin were used to determine the stereospecificity of topoisomerase II for the C-11 methyl group in tricyclic quinolones. In all cases, the S isomer was the most active compound against the eukaryotic enzyme. It was approximately 2.2-fold more potent than the R isomer at inhibiting the overall catalytic activity of topoisomerase II (as monitored by DNA relaxation assays). A markedly greater difference in quinolone activity was observed in enzyme-mediated DNA cleavage reactions. While the S enantiomer stimulated nucleic acid breakage approximately 3.5-fold, the R compound did not enhance and, in fact, decreased initial DNA cleavage levels by approximately 50%. The activity of the racemic mixture more closely resembled that of the R enantiomer. In competition experiments, the DNA cleavage-enhancing effects of the S isomer were attenuated by the R compound. Taken together, these latter results indicate that the R enantiomer is an antagonist of S isomer-promoted topoisomerase II-mediated DNA cleavage. Finally, the cytotoxic potential of quinolyl-substituted ofloxacin analogs correlated with the ability to stimulate enzyme-mediated DNA cleavage. Thus, stereochemistry appears to be a governing factor for the potential development of tricyclic quinolones as topoisomerase II-targeted drugs with antineoplastic activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. F., Gootz T. D., McGuirk P. R., Farrell C. A., Sokolowski S. A. Use of in vitro topoisomerase II assays for studying quinolone antibacterial agents. Antimicrob Agents Chemother. 1989 Oct;33(10):1697–1703. doi: 10.1128/aac.33.10.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsea S. H., Osheroff N., Nitiss J. L. Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast. J Biol Chem. 1992 Jul 5;267(19):13150–13153. [PubMed] [Google Scholar]
- Fujimoto T., Mitsuhashi S. In vitro antibacterial activity of DR-3355, the S-(-)-isomer of ofloxacin. Chemotherapy. 1990;36(4):268–276. doi: 10.1159/000238777. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster J. F., Rohlfing S. R., Pecore S. E., Winandy R. M., Stern R. M., Landmesser J. E., Olsen R. A., Gleason W. B. Synthesis, absolute configuration, and antibacterial activity of 6,7-dihydro-5,8-dimethyl-9-fluoro-1-oxo-1H,5H- benzo[ij]quinolizine-2-carboxylic acid. J Med Chem. 1987 May;30(5):839–843. doi: 10.1021/jm00388a016. [DOI] [PubMed] [Google Scholar]
- Hayakawa I., Atarashi S., Yokohama S., Imamura M., Sakano K., Furukawa M. Synthesis and antibacterial activities of optically active ofloxacin. Antimicrob Agents Chemother. 1986 Jan;29(1):163–164. doi: 10.1128/aac.29.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Hoshino K., Sato K., Akahane K., Yoshida A., Hayakawa I., Sato M., Une T., Osada Y. Significance of the methyl group on the oxazine ring of ofloxacin derivatives in the inhibition of bacterial and mammalian type II topoisomerases. Antimicrob Agents Chemother. 1991 Feb;35(2):309–312. doi: 10.1128/aac.35.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K., Sato K., Une T., Osada Y. Inhibitory effects of quinolones on DNA gyrase of Escherichia coli and topoisomerase II of fetal calf thymus. Antimicrob Agents Chemother. 1989 Oct;33(10):1816–1818. doi: 10.1128/aac.33.10.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussy P., Maass G., Tümmler B., Grosse F., Schomburg U. Effect of 4-quinolones and novobiocin on calf thymus DNA polymerase alpha primase complex, topoisomerases I and II, and growth of mammalian lymphoblasts. Antimicrob Agents Chemother. 1986 Jun;29(6):1073–1078. doi: 10.1128/aac.29.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura M., Shibamura S., Hayakawa I., Osada Y. Inhibition of DNA gyrase by optically active ofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):325–327. doi: 10.1128/aac.31.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlbrenner W. E., Wideburg N., Weigl D., Saldivar A., Chu D. T. Induction of calf thymus topoisomerase II-mediated DNA breakage by the antibacterial isothiazoloquinolones A-65281 and A-65282. Antimicrob Agents Chemother. 1992 Jan;36(1):81–86. doi: 10.1128/aac.36.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Mitscher L. A., Sharma P. N., Chu D. T., Shen L. L., Pernet A. G. Chiral DNA gyrase inhibitors. 2. Asymmetric synthesis and biological activity of the enantiomers of 9-fluoro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-2,3-dihydro-7H- pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid (ofloxacin). J Med Chem. 1987 Dec;30(12):2283–2286. doi: 10.1021/jm00395a017. [DOI] [PubMed] [Google Scholar]
- Moreau N. J., Robaux H., Baron L., Tabary X. Inhibitory effects of quinolones on pro- and eucaryotic DNA topoisomerases I and II. Antimicrob Agents Chemother. 1990 Oct;34(10):1955–1960. doi: 10.1128/aac.34.10.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomori Y., Yasue T., Aoyama H., Hirai K., Suzue S., Yokota T. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J Antimicrob Chemother. 1988 Oct;22 (Suppl 500):91–97. doi: 10.1093/jac/22.supplement_d.91. [DOI] [PubMed] [Google Scholar]
- Osheroff N. Eukaryotic topoisomerase II. Characterization of enzyme turnover. J Biol Chem. 1986 Jul 25;261(21):9944–9950. [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Osheroff N., Zechiedrich E. L., Gale K. C. Catalytic function of DNA topoisomerase II. Bioessays. 1991 Jun;13(6):269–273. doi: 10.1002/bies.950130603. [DOI] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Robinson M. J., Martin B. A., Gootz T. D., McGuirk P. R., Moynihan M., Sutcliffe J. A., Osheroff N. Effects of quinolone derivatives on eukaryotic topoisomerase II. A novel mechanism for enhancement of enzyme-mediated DNA cleavage. J Biol Chem. 1991 Aug 5;266(22):14585–14592. [PubMed] [Google Scholar]
- Robinson M. J., Martin B. A., Gootz T. D., McGuirk P. R., Osheroff N. Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of the C-8 fluorine group. Antimicrob Agents Chemother. 1992 Apr;36(4):751–756. doi: 10.1128/aac.36.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. J., Osheroff N. Effects of antineoplastic drugs on the post-strand-passage DNA cleavage/religation equilibrium of topoisomerase II. Biochemistry. 1991 Feb 19;30(7):1807–1813. doi: 10.1021/bi00221a012. [DOI] [PubMed] [Google Scholar]
- Sanchez J. P., Domagala J. M., Heifetz C. L., Priebe S. R., Sesnie J. A., Trehan A. K. Quinolone antibacterial agents. Synthesis and structure-activity relationships of a series of amino acid prodrugs of racemic and chiral 7-(3-amino-1-pyrrolidinyl)quinolones. Highly soluble quinolone prodrugs with in vivo pseudomonas activity. J Med Chem. 1992 May 15;35(10):1764–1773. doi: 10.1021/jm00088a011. [DOI] [PubMed] [Google Scholar]
- Schneider E., Hsiang Y. H., Liu L. F. DNA topoisomerases as anticancer drug targets. Adv Pharmacol. 1990;21:149–183. doi: 10.1016/s1054-3589(08)60342-7. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Osheroff N., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Purification and physical characterization. J Biol Chem. 1983 Aug 10;258(15):9530–9535. [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. A., Gootz T. D., Barrett J. F. Biochemical characteristics and physiological significance of major DNA topoisomerases. Antimicrob Agents Chemother. 1989 Dec;33(12):2027–2033. doi: 10.1128/aac.33.12.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landuyt H. W., Magerman K., Gordts B. The importance of the quinolones in antibacterial therapy. J Antimicrob Chemother. 1990 Nov;26 (Suppl 500):1–6. doi: 10.1093/jac/26.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Ashizawa T., Morimoto M., Hosomi J., Nakano H. Antitumor quinolones with mammalian topoisomerase II mediated DNA cleavage activity. Cancer Res. 1992 May 15;52(10):2818–2822. [PubMed] [Google Scholar]
- Zimmer C., Störl K., Störl J. Microbial DNA topoisomerases and their inhibition by antibiotics. J Basic Microbiol. 1990;30(3):209–224. doi: 10.1002/jobm.3620300312. [DOI] [PubMed] [Google Scholar]