Abstract
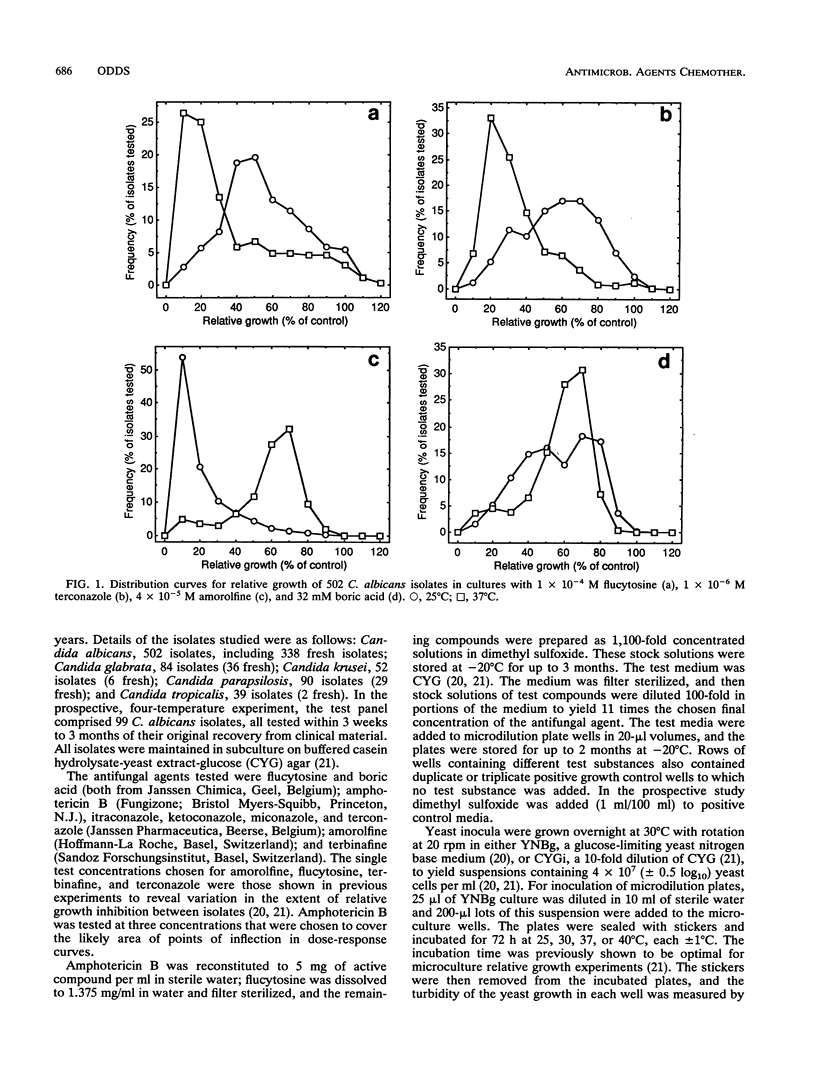
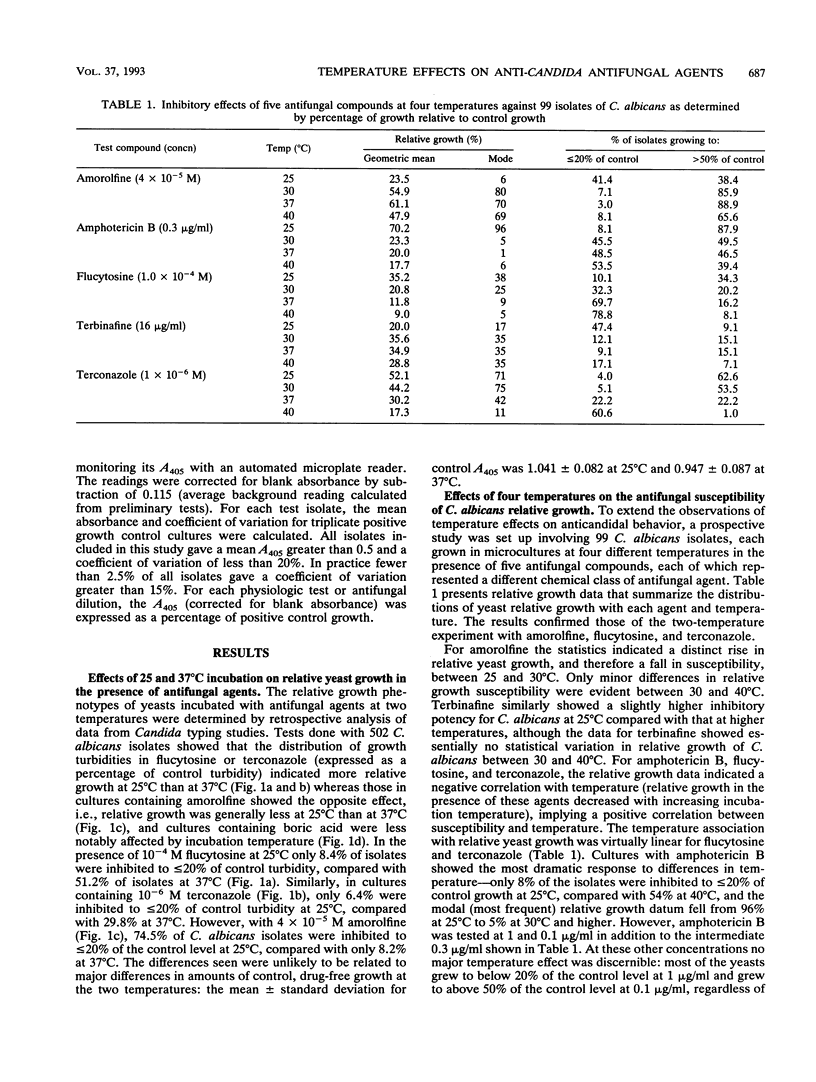
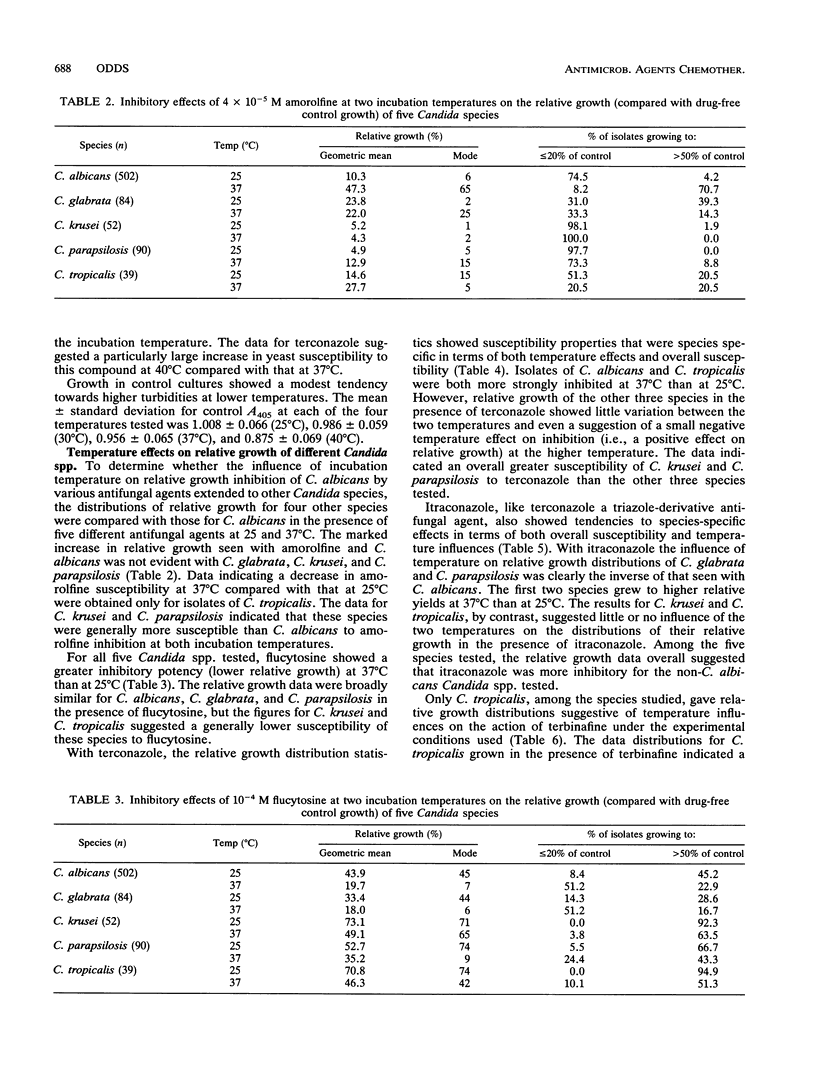
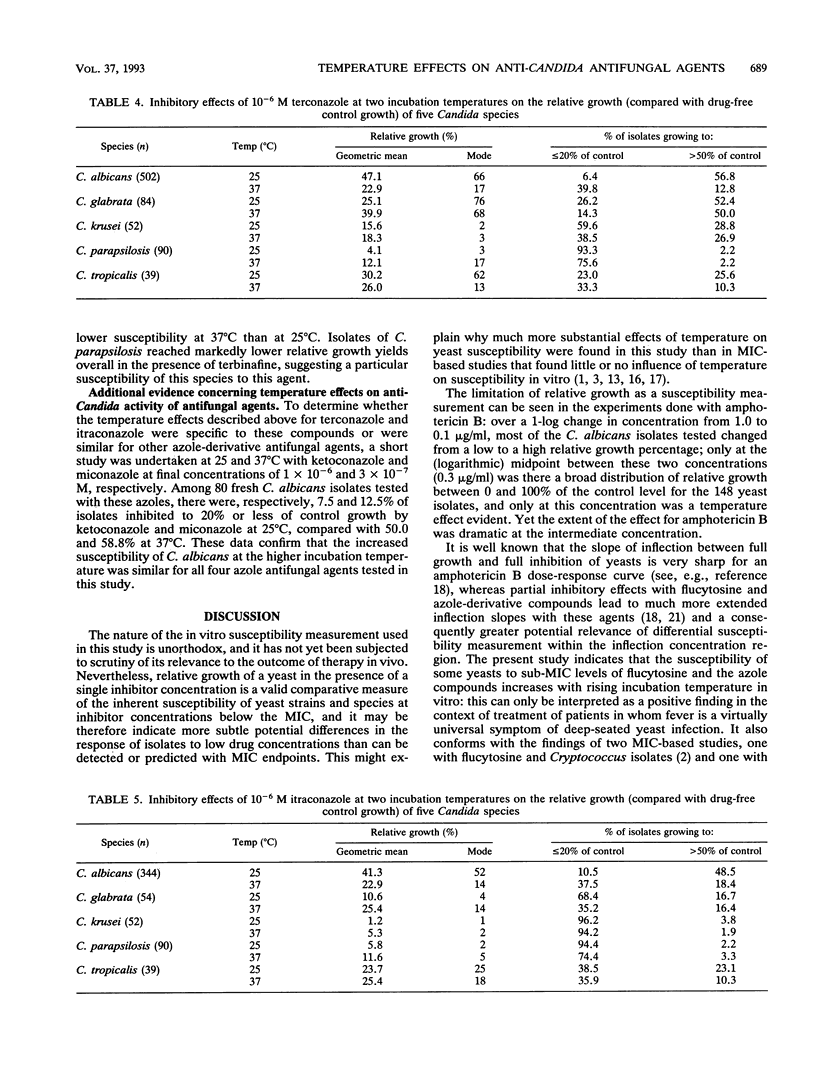
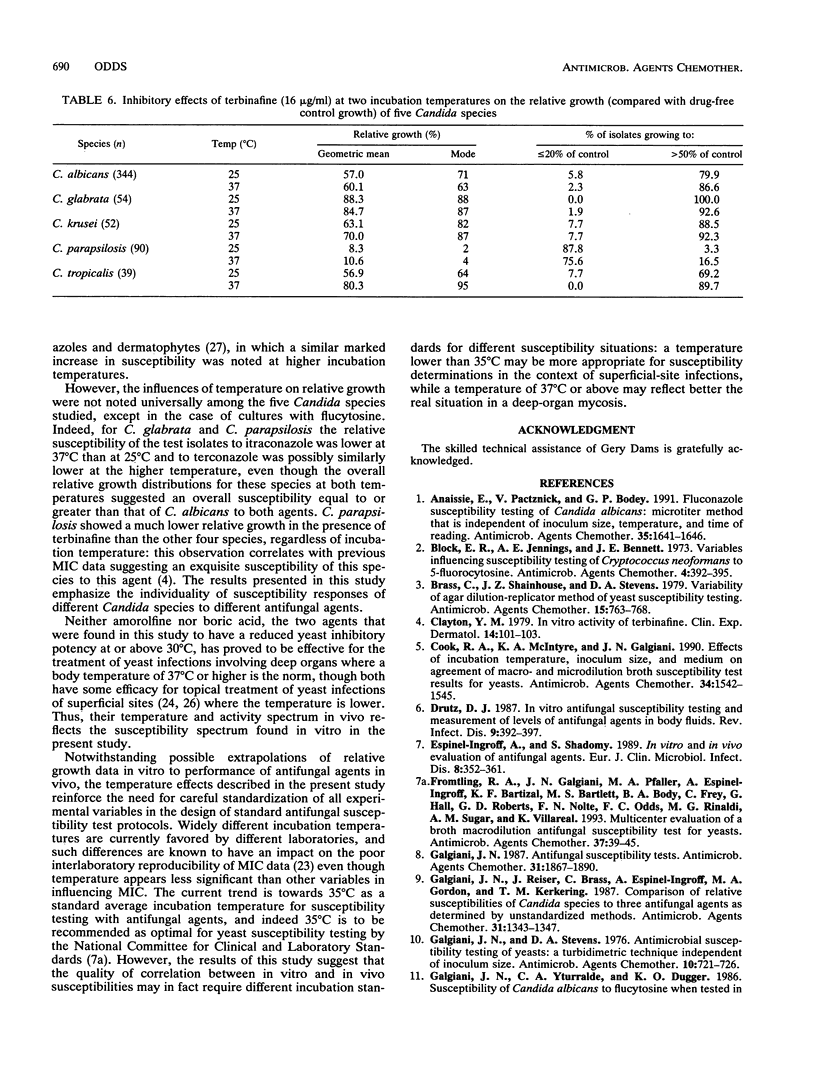
The relative growth (percentage of growth relative to control growth) of 767 Candida isolates representing five species was measured in microcultures at 25 and 37 degrees C. In the presence of 10(-4) M flucytosine, the distribution of relative yeast growth data indicated that Candida albicans isolates were less susceptible at 25 degrees C than at 37 degrees C, while the opposite was found with 4 x 10(-5) M amorolfine for most of the isolates tested. Repetition of the experiments at four different temperatures with 99 C. albicans isolates and five antifungal agents confirmed a direct relationship between growth inhibition and increasing temperature from 25 to 40 degrees C with amphotericin B, flucytosine, and terconazole; a strong inverse relationship between inhibition and temperature with amorolfine; and a weak inverse relationship with terbinafine. However, these relationships were not always noted with other Candida spp.: in particular, the growth of C. glabrata and C. parapsilosis isolates tended to be greater at 37 degrees C than at 25 degrees C in the presence of the azole-derivative antifungal agents itraconazole and terconazole. These findings stress the species-specific individuality of yeast susceptibility to azole antifungal agents. The results with C. albicans and amorolfine and terbinafine accord with their known in vivo efficacy in mycoses involving low-temperature superficial sites and poor activity against mycoses involving deep body sites. The data also reinforce the need for control of experimental variables such as temperature in the design of standardized yeast susceptibility tests.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anaissie E., Paetznick V., Bodey G. P. Fluconazole susceptibility testing of Candida albicans: microtiter method that is independent of inoculum size, temperature, and time of reading. Antimicrob Agents Chemother. 1991 Aug;35(8):1641–1646. doi: 10.1128/aac.35.8.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block E. R., Jennings A. E., Bennett J. E. Variables influencing susceptibility testing of Cryptococcus neoformans to 5-fluorocytosine. Antimicrob Agents Chemother. 1973 Oct;4(4):392–395. doi: 10.1128/aac.4.4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass C., Shainhouse J. Z., Stevens D. A. Variability of agar dilution-replicator method of yeast susceptibility testing. Antimicrob Agents Chemother. 1979 Jun;15(6):763–768. doi: 10.1128/aac.15.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton Y. M. In vitro activity of terbinafine. Clin Exp Dermatol. 1989 Mar;14(2):101–103. doi: 10.1111/j.1365-2230.1989.tb00901.x. [DOI] [PubMed] [Google Scholar]
- Cook R. A., McIntyre K. A., Galgiani J. N. Effects of incubation temperature, inoculum size, and medium on agreement of macro- and microdilution broth susceptibility test results for yeasts. Antimicrob Agents Chemother. 1990 Aug;34(8):1542–1545. doi: 10.1128/aac.34.8.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz D. J. In vitro antifungal susceptibility testing and measurement of levels of antifungal agents in body fluids. Rev Infect Dis. 1987 Mar-Apr;9(2):392–397. doi: 10.1093/clinids/9.2.392. [DOI] [PubMed] [Google Scholar]
- Espinel-Ingroff A., Shadomy S. In vitro and in vivo evaluation of antifungal agents. Eur J Clin Microbiol Infect Dis. 1989 Apr;8(4):352–361. doi: 10.1007/BF01963469. [DOI] [PubMed] [Google Scholar]
- Fromtling R. A., Galgiani J. N., Pfaller M. A., Espinel-Ingroff A., Bartizal K. F., Bartlett M. S., Body B. A., Frey C., Hall G., Roberts G. D. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob Agents Chemother. 1993 Jan;37(1):39–45. doi: 10.1128/aac.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N. Antifungal susceptibility tests. Antimicrob Agents Chemother. 1987 Dec;31(12):1867–1870. doi: 10.1128/aac.31.12.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Reiser J., Brass C., Espinel-Ingroff A., Gordon M. A., Kerkering T. M. Comparison of relative susceptibilities of Candida species to three antifungal agents as determined by unstandardized methods. Antimicrob Agents Chemother. 1987 Sep;31(9):1343–1347. doi: 10.1128/aac.31.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Stevens D. A. Antimicrobial susceptibility testing of yeasts: a turbidimetric technique independent of inoculum size. Antimicrob Agents Chemother. 1976 Oct;10(4):721–728. doi: 10.1128/aac.10.4.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgiani J. N., Yturralde C. A., Dugger K. O. Susceptibility of Candida albicans to flucytosine when tested in different formulations of yeast nitrogen base broth. Diagn Microbiol Infect Dis. 1986 Sep;5(3):273–276. doi: 10.1016/0732-8893(86)90012-x. [DOI] [PubMed] [Google Scholar]
- Gordon M. A., Lapa E. W., Passero P. G. Improved method for azole antifungal susceptibility testing. J Clin Microbiol. 1988 Sep;26(9):1874–1877. doi: 10.1128/jcm.26.9.1874-1877.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. J., Newman R. L. Laboratory assessment of the antimycotic drug clotrimazole. J Clin Pathol. 1972 Dec;25(12):1089–1097. doi: 10.1136/jcp.25.12.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B., White R. J., Williamson G. M. Factors influencing the susceptibility of Candida albicans to the polyenoic antibiotics nystatin and amphotericin B. J Gen Microbiol. 1978 Feb;104(2):325–333. doi: 10.1099/00221287-104-2-325. [DOI] [PubMed] [Google Scholar]
- Kobayashi G. S., Spitzer E. D. Testing of organisms for susceptibility to triazoles: is it justified? Eur J Clin Microbiol Infect Dis. 1989 May;8(5):387–389. doi: 10.1007/BF01964051. [DOI] [PubMed] [Google Scholar]
- Mazens M. F., Andrews G. P., Bartlett R. C. Comparison of microdilution and broth dilution techniques for the susceptibility testing of yeasts to 5-fluorocytosine and amphotericin B. Antimicrob Agents Chemother. 1979 Mar;15(3):475–477. doi: 10.1128/aac.15.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre K. A., Galgiani J. N. In vitro susceptibilities of yeasts to a new antifungal triazole, SCH 39304: effects of test conditions and relation to in vivo efficacy. Antimicrob Agents Chemother. 1989 Jul;33(7):1095–1100. doi: 10.1128/aac.33.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C., Abbott A. B. Relative inhibition factors--a novel approach to the assessment of antifungal antibiotics in vitro. J Antimicrob Chemother. 1984 Jan;13(1):31–43. doi: 10.1093/jac/13.1.31. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Antifungal susceptibility testing of Candida spp. by relative growth measurement at single concentrations of antifungal agents. Antimicrob Agents Chemother. 1992 Aug;36(8):1727–1737. doi: 10.1128/aac.36.8.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Problems in the laboratory assessment of antifungal activity. Postgrad Med J. 1979 Sep;55(647):677–680. doi: 10.1136/pgmj.55.647.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds F. C. Quantitative microculture system with standardized inocula for strain typing, susceptibility testing, and other physiologic measurements with Candida albicans and other yeasts. J Clin Microbiol. 1991 Dec;29(12):2735–2740. doi: 10.1128/jcm.29.12.2735-2740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Rinaldi M. G., Galgiani J. N., Bartlett M. S., Body B. A., Espinel-Ingroff A., Fromtling R. A., Hall G. S., Hughes C. E., Odds F. C. Collaborative investigation of variables in susceptibility testing of yeasts. Antimicrob Agents Chemother. 1990 Sep;34(9):1648–1654. doi: 10.1128/aac.34.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radetsky M., Wheeler R. C., Roe M. H., Todd J. K. Microtiter broth dilution method for yeast susceptibility testing with validation by clinical outcome. J Clin Microbiol. 1986 Oct;24(4):600–606. doi: 10.1128/jcm.24.4.600-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swate T. E., Weed J. C. Boric acid treatment of vulvovaginal candidiasis. Obstet Gynecol. 1974 Jun;43(6):893–895. [PubMed] [Google Scholar]
- Wright L. R., Scott E. M., Gorman S. P. The sensitivity of mycelium, arthrospores and microconidia of Trichophyton mentagrophytes to imidazoles determined by in-vitro tests. J Antimicrob Chemother. 1983 Oct;12(4):317–327. doi: 10.1093/jac/12.4.317. [DOI] [PubMed] [Google Scholar]


