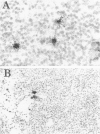
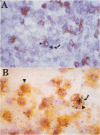
Abstract
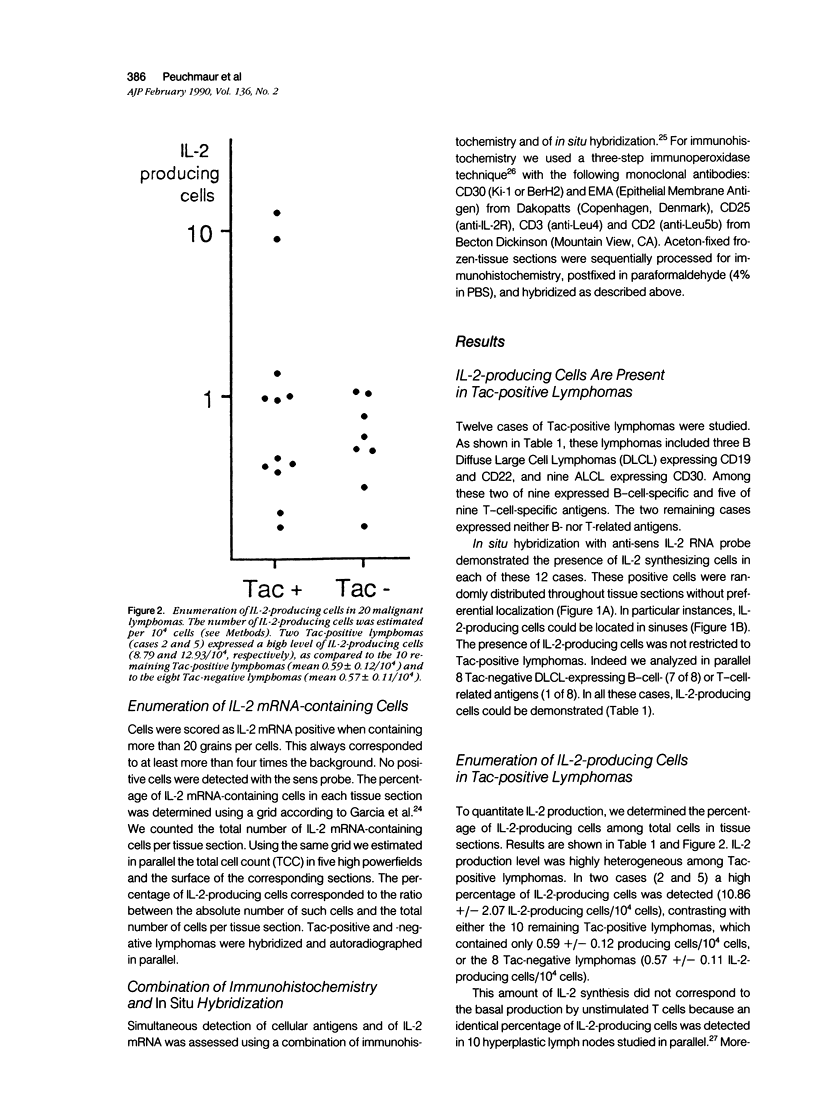
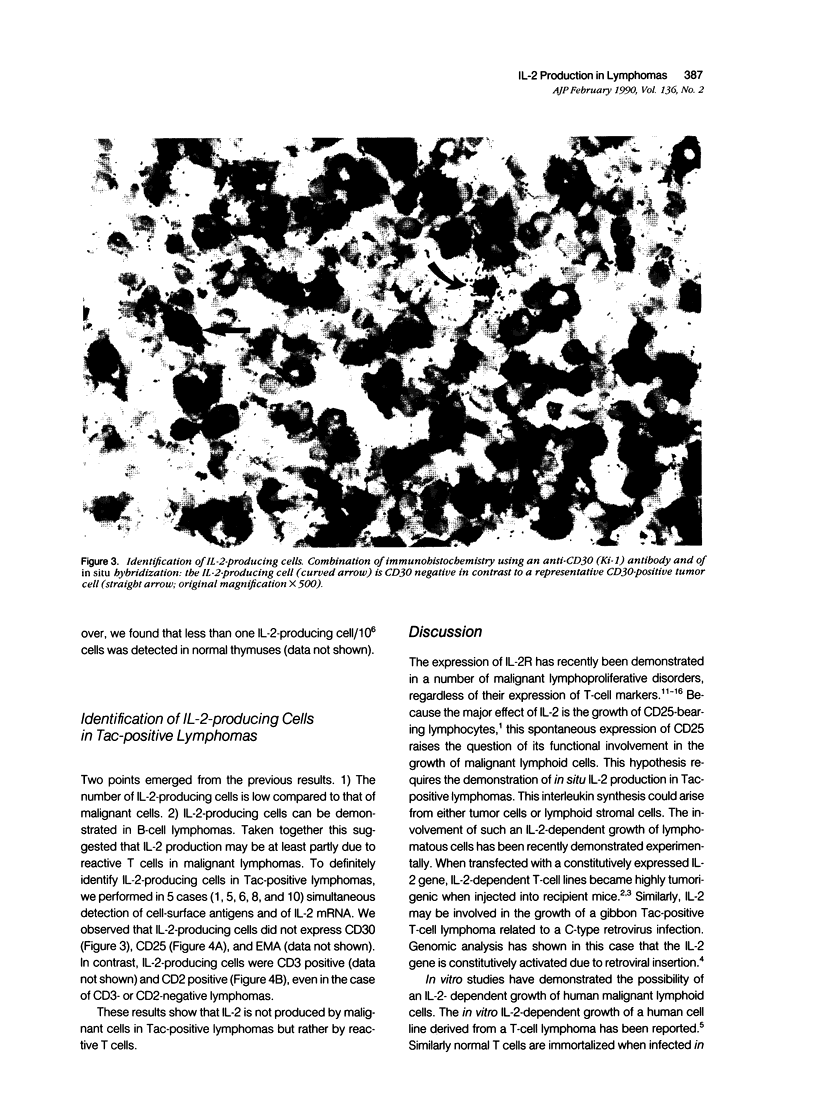
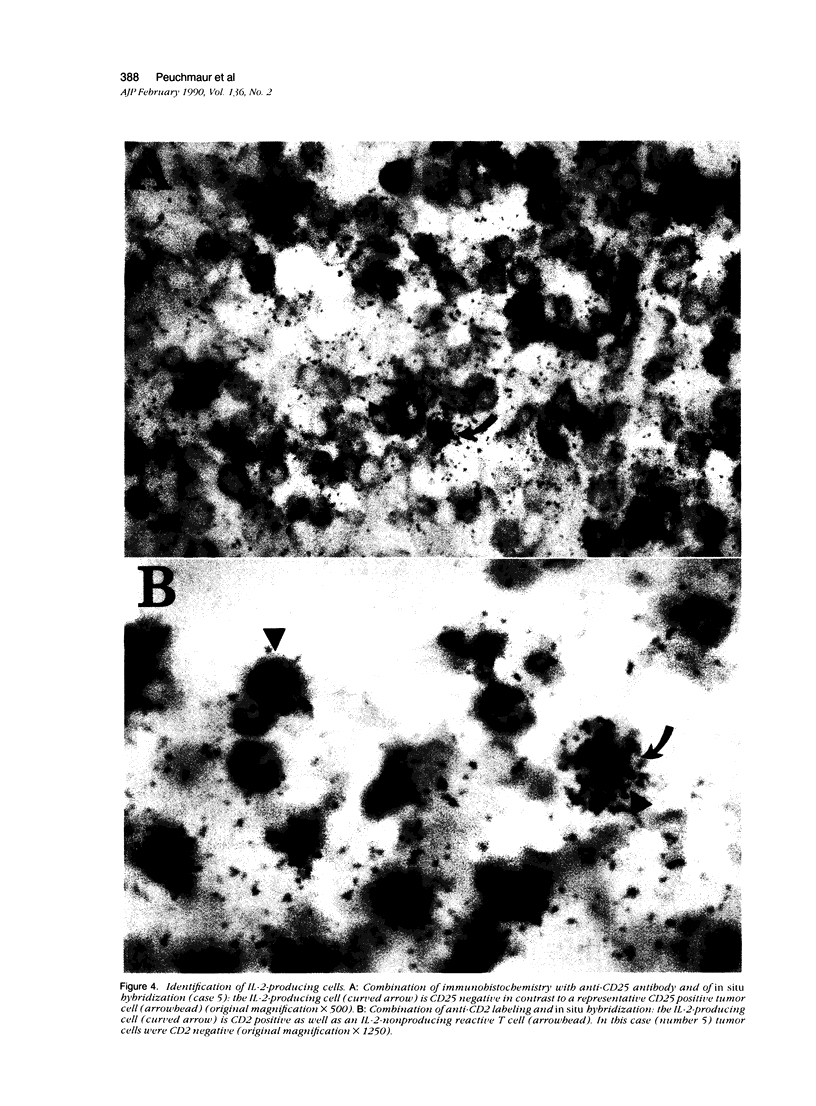
Expression of the IL-2 receptor (Tac antigen/CD25) is documented in malignant lymphomas. Because IL-2 is a major lymphocyte growth factor, an IL-2-dependent growth could be involved in the proliferation of Tac-positive lymphomas. Indeed such a mechanism has been demonstrated experimentally for the growth of T-cell lines. To investigate this point in human lymphomas, we used in situ hybridization to analyze the expression of the IL-2 gene in 20 non-Hodgkin's lymphomas, among which 12 expressed the IL-2 receptor. Nine of these were anaplastic large cell lymphomas expressing the Ki-1-related antigen. We here show that IL-2-producing cells are present in all the lymphomas we analyzed. As a mean, there is no significant difference in the percentage of IL-2-producing cells between Tac-positive and -negative lymphomas. However, the level of IL-2 production is highly heterogeneous in both groups, and the highest density of IL-2-producing cells was observed in 2 Tac-positive lymphomas. Simultaneous detection of cellular antigens and of IL-2 mRNA demonstrates that IL-2 is produced by reactive T cells rather than by tumor cells. These results suggest that if IL-2 is involved in the growth of Tac-positive lymphomas, it acts as a paracrine, rather than an autocrine, factor.
Full text
PDF
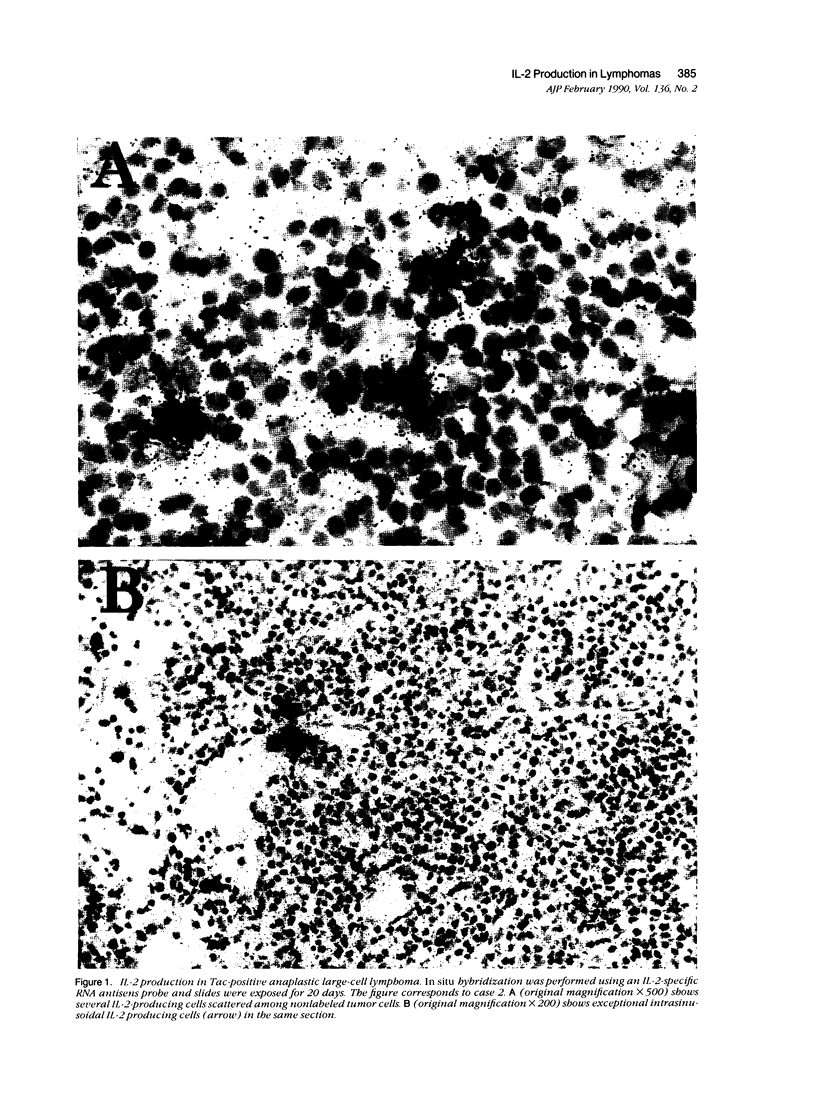


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnarsson B. A., Kadin M. E. Ki-1 positive large cell lymphoma. A morphologic and immunologic study of 19 cases. Am J Surg Pathol. 1988 Apr;12(4):264–274. doi: 10.1097/00000478-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Arima N., Daitoku Y., Ohgaki S., Fukumori J., Tanaka H., Yamamoto Y., Fujimoto K., Onoue K. Autocrine growth of interleukin 2-producing leukemic cells in a patient with adult T cell leukemia. Blood. 1986 Sep;68(3):779–782. [PubMed] [Google Scholar]
- Arya S. K., Wong-Staal F., Gallo R. C. T-cell growth factor gene: lack of expression in human T-cell leukemia-lymphoma virus-infected cells. Science. 1984 Mar 9;223(4640):1086–1087. doi: 10.1126/science.6320374. [DOI] [PubMed] [Google Scholar]
- Bonnefoix T., Piccinni M. P., Jacob M. C., Pegourie B., Sotto J. J. Limiting dilution analysis of the frequency of IL2 responsive T cells in lymph nodes involved by B-cell non-Hodgkin's lymphomas. Leuk Res. 1989;13(4):323–329. doi: 10.1016/0145-2126(89)90069-6. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T., Cash E. Simultaneous in situ detection of viral RNA and antigens. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5445–5448. doi: 10.1073/pnas.81.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delsol G., Al Saati T., Gatter K. C., Gerdes J., Schwarting R., Caveriviere P., Rigal-Huguet F., Robert A., Stein H., Mason D. Y. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988 Jan;130(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Duprez V., Lenoir G., Dautry-Varsat A. Autocrine growth stimulation of a human T-cell lymphoma line by interleukin 2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6932–6936. doi: 10.1073/pnas.82.20.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D. B., Kamoun M., Norris C. A., Holbrook N. J., Greengard J. S., Crabtree G. R., Kant J. A. Retroviral activation of interleukin 2 gene in a gibbon ape T cell lymphoma line. J Exp Med. 1986 Nov 1;164(5):1723–1734. doi: 10.1084/jem.164.5.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilie D., Peuchmaur M., Barad M., Jouin H., Maillot M. C., Couez D., Nicolas J. F., Malissen B. Visualizing interleukin 2 gene expression at the single cell level. Eur J Immunol. 1989 Sep;19(9):1619–1624. doi: 10.1002/eji.1830190915. [DOI] [PubMed] [Google Scholar]
- Erber W. N., Mason D. Y. Expression of the interleukin-2 receptor (Tac antigen/CD25) in hematologic neoplasms. Am J Clin Pathol. 1988 May;89(5):645–648. doi: 10.1093/ajcp/89.5.645. [DOI] [PubMed] [Google Scholar]
- Froese P., Lemke H., Gerdes J., Havsteen B., Schwarting R., Hansen H., Stein H. Biochemical characterization and biosynthesis of the Ki-1 antigen in Hodgkin-derived and virus-transformed human B and T lymphoid cell lines. J Immunol. 1987 Sep 15;139(6):2081–2087. [PubMed] [Google Scholar]
- Gallo R. C., Wong-Staal F. Retroviruses as etiologic agents of some animal and human leukemias and lymphomas and as tools for elucidating the molecular mechanism of leukemogenesis. Blood. 1982 Sep;60(3):545–557. [PubMed] [Google Scholar]
- Garcia C. F., Weiss L. M., Lowder J., Komoroske C., Link M. P., Levy R., Warnke R. A. Quantitation and estimation of lymphocyte subsets in tissue sections. Comparison with flow cytometry. Am J Clin Pathol. 1987 Apr;87(4):470–477. doi: 10.1093/ajcp/87.4.470. [DOI] [PubMed] [Google Scholar]
- Goebels N., Waase I., Pfizenmaier K., Krönke M. IL-2 production in human T lymphotropic virus I-infected leukemic T lymphocytes analyzed by in situ hybridization. J Immunol. 1988 Aug 15;141(4):1231–1235. [PubMed] [Google Scholar]
- Granelli-Piperno A. In situ hybridization for interleukin 2 and interleukin 2 receptor mRNA in T cells activated in the presence or absence of cyclosporin A. J Exp Med. 1988 Nov 1;168(5):1649–1658. doi: 10.1084/jem.168.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant B. W., Platt J. L., Jacob H. S., Kay N. E. Lymphocyte populations and TAC-antigen in diffuse B-cell lymphomas. Leuk Res. 1986;10(11):1271–1278. doi: 10.1016/0145-2126(86)90333-4. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Karasuyama H., Tohyama N., Tada T. Autocrine growth and tumorigenicity of interleukin 2-dependent helper T cells transfected with IL-2 gene. J Exp Med. 1989 Jan 1;169(1):13–25. doi: 10.1084/jem.169.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kradin R. L., Kurnick J. T., Lazarus D. S., Preffer F. I., Dubinett S. M., Pinto C. E., Gifford J., Davidson E., Grove B., Callahan R. J. Tumour-infiltrating lymphocytes and interleukin-2 in treatment of advanced cancer. Lancet. 1989 Mar 18;1(8638):577–580. doi: 10.1016/s0140-6736(89)91609-7. [DOI] [PubMed] [Google Scholar]
- Laurent G., Al Saati T., Olive D., Laurent J. C., Poncelet P., Delsol G. Expression of Tac antigen in B cell lymphomas. Clin Exp Immunol. 1986 Aug;65(2):354–362. [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Sammons R. E. The labeled antigen method of immunoenzymatic staining. J Histochem Cytochem. 1979 Apr;27(4):832–840. doi: 10.1177/27.4.109496. [DOI] [PubMed] [Google Scholar]
- Nawrocki J. F., Kirsten E. S., Fisher R. I. Biochemical and structural properties of a Hodgkin's disease-related membrane protein. J Immunol. 1988 Jul 15;141(2):672–680. [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Winberg C. D., van de Velde S., Blayney D. W., Rappaport H. Distribution of lymphocytes with interleukin-2 receptors (TAC antigens) in reactive lymphoproliferative processes, Hodgkin's disease, and non-Hodgkin's lymphomas. An immunohistologic study of 300 cases. Am J Pathol. 1987 Apr;127(1):27–37. [PMC free article] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Strauchen J. A., Breakstone B. A. IL-2 receptor expression in human lymphoid lesions. Immunohistochemical study of 166 cases. Am J Pathol. 1987 Mar;126(3):506–512. [PMC free article] [PubMed] [Google Scholar]
- Waldmann T. A., Goldman C. K., Bongiovanni K. F., Sharrow S. O., Davey M. P., Cease K. B., Greenberg S. J., Longo D. L. Therapy of patients with human T-cell lymphotrophic virus I-induced adult T-cell leukemia with anti-Tac, a monoclonal antibody to the receptor for interleukin-2. Blood. 1988 Nov;72(5):1805–1816. [PubMed] [Google Scholar]
- Waldmann T. A. The structure, function, and expression of interleukin-2 receptors on normal and malignant lymphocytes. Science. 1986 May 9;232(4751):727–732. doi: 10.1126/science.3008337. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Michie S. A., Medeiros L. J., Strickler J. G., Garcia C. F., Warnke R. A. Expression of Tac antigen by non-Hodgkin's lymphomas. Am J Clin Pathol. 1987 Oct;88(4):483–485. doi: 10.1093/ajcp/88.4.483. [DOI] [PubMed] [Google Scholar]
- Yamada G., Kitamura Y., Sonoda H., Harada H., Taki S., Mulligan R. C., Osawa H., Diamantstein T., Yokoyama S., Taniguchi T. Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J. 1987 Sep;6(9):2705–2709. doi: 10.1002/j.1460-2075.1987.tb02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]