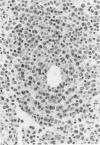
Abstract
In contrast to its role in B-lymphomagenesis, Epstein-Barr Virus (EBV) only incidentally has been associated with T-cell lymphomas. In the present report we describe a fourth patient with EBV-related T-cell lymphoma. The patient presented with an angio-immunoblastic lymphadenopathy (AILD)-like T-cell lymphoma. Serology was compatible with chronic Epstein-Barr (EBV) infection. After a 1-year period of waxing and waning lymphadenopathy, this lymphoma evolved to an aggressive CD8+ Immunoblastic T-cell lymphoma. A relationship with the chronic EBV infection was indicated by the finding of EBV genome in the tumor tissue by Southern blot analysis. Moreover, EBV nuclear antigen (EBNA) was detected in situ within individually defined CD8+ tumor cells by two-color immunofluorescence. Two alternative possibilities, namely that EBV primarily played a role in lymphomagenesis of the AILD-like T-cell lymphoma or that the virus was an additional oncogenic event in the final process of tumor progression to the immunoblastic lymphoma, are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom R., Geelen J. L., Sol C. J., Raap A. K., Minnaar R. P., Klaver B. P., van der Noordaa J. Establishment of a rat cell line inducible for the expression of human cytomegalovirus immediate-early gene products by protein synthesis inhibition. J Virol. 1986 Jun;58(3):851–859. doi: 10.1128/jvi.58.3.851-859.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros I., Pósfai G., Venetianer P. High-copy-number derivatives of the plasmid cloning vector pBR322. Gene. 1984 Oct;30(1-3):257–260. doi: 10.1016/0378-1119(84)90130-6. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Nalesnik M. A., Shearer W. T., Sklar J. Clonal analysis of transplant-associated lymphoproliferations based on the structure of the genomic termini of the Epstein-Barr virus. Blood. 1988 Jul;72(1):349–352. [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Fischer D. K., Miller G., Gradoville L., Heston L., Westrate M. W., Maris W., Wright J., Brandsma J., Summers W. C. Genome of a mononucleosis Epstein-Barr virus contains DNA fragments previously regarded to be unique to Burkitt's lymphoma isolates. Cell. 1981 May;24(2):543–553. doi: 10.1016/0092-8674(81)90345-7. [DOI] [PubMed] [Google Scholar]
- Hanto D. W., Frizzera G., Purtilo D. T., Sakamoto K., Sullivan J. L., Saemundsen A. K., Klein G., Simmons R. L., Najarian J. S. Clinical spectrum of lymphoproliferative disorders in renal transplant recipients and evidence for the role of Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4253–4261. [PubMed] [Google Scholar]
- Jones J. F., Shurin S., Abramowsky C., Tubbs R. R., Sciotto C. G., Wahl R., Sands J., Gottman D., Katz B. Z., Sklar J. T-cell lymphomas containing Epstein-Barr viral DNA in patients with chronic Epstein-Barr virus infections. N Engl J Med. 1988 Mar 24;318(12):733–741. doi: 10.1056/NEJM198803243181203. [DOI] [PubMed] [Google Scholar]
- Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc Natl Acad Sci U S A. 1979 May;76(5):2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluin P. M., Slootweg P. J., Schuurman H. J., Go D. M., Rademakers L. H., van der Putte S. C., van Unnik J. A. Primary B-cell malignant lymphoma of the maxilla with a sarcomatous pattern and multilobated nuclei. Cancer. 1984 Oct 15;54(8):1598–1605. doi: 10.1002/1097-0142(19841015)54:8<1598::aid-cncr2820540822>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kuis W., Roord J. J., Zegers B. J., Rickinson A. B., Kapsenberg J. G., The H., Stoop J. W. Heterogeneity of immune defects in three children with a chronic active Epstein-Barr virus infection. J Clin Immunol. 1985 Nov;5(6):377–385. doi: 10.1007/BF00915334. [DOI] [PubMed] [Google Scholar]
- Leyvraz S., Henle W., Chahinian A. P., Perlmann C., Klein G., Gordon R. E., Rosenblum M., Holland J. F. Association of Epstein-Barr virus with thymic carcinoma. N Engl J Med. 1985 May 16;312(20):1296–1299. doi: 10.1056/NEJM198505163122006. [DOI] [PubMed] [Google Scholar]
- Ono A., Ueda T. Synthesis of decadeoxyribonucleotides containing N6-methyladenine, N4-methylcytosine, and 5-methylcytosine: recognition and cleavage by restriction endonucleases (nucleosides and nucleotides part 74). Nucleic Acids Res. 1987 Jan 12;15(1):219–232. doi: 10.1093/nar/15.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulutke M., Khilanani P., Weise R. Immunologic and electronmicroscopic characteristics of a case of immunoblastic lymphadenopathy. Am J Clin Pathol. 1976 Jun;65(6):929–941. doi: 10.1093/ajcp/65.6.929. [DOI] [PubMed] [Google Scholar]
- Purtilo D. T., Liao S. A., Sakamoto K., Snyder L. M., DeFlorio D., Jr, Bhawan J., Paquin L., Yang J. P., Hutt-Fletcher L. M., Muralidharan K. Diverse familial malignant tumors and Epstein-Barr virus. Cancer Res. 1981 Nov;41(11 Pt 1):4248–4252. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saemundsen A. K., Berkel A. I., Henle W., Henle G., Anvret M., Sanal O., Ersoy F., Cağlar M., Klein G. Epstein-Barr-virus-carrying lymphoma in a patient with ataxia-telangiectasia. Br Med J (Clin Res Ed) 1981 Feb 7;282(6262):425–427. doi: 10.1136/bmj.282.6262.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore A., Dosch H. M., Gelfand E. W. Expression and modulation of C3 receptors during early T-cell ontogeny. Cell Immunol. 1979 Jun;45(1):157–166. doi: 10.1016/0008-8749(79)90371-x. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Lemon S. M., Pagano J. S. A second site for Epstein-Barr virus shedding: the uterine cervix. Lancet. 1986 Nov 15;2(8516):1122–1124. doi: 10.1016/s0140-6736(86)90531-3. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Nedrud J. G., Raab-Traub N., Hanes R. A., Pagano J. S. Epstein-Barr virus replication in oropharyngeal epithelial cells. N Engl J Med. 1984 May 10;310(19):1225–1230. doi: 10.1056/NEJM198405103101905. [DOI] [PubMed] [Google Scholar]
- Tobi M., Straus S. E. Chronic Epstein-Barr virus disease: a workshop held by the National Institute of Allergy and Infectious Diseases. Ann Intern Med. 1985 Dec;103(6 ):951–953. doi: 10.7326/0003-4819-103-6-951. [DOI] [PubMed] [Google Scholar]
- Virelizier J. L., Lenoir G., Griscelli C. Persistent Epstein-Barr virus infection in a child with hypergammaglobulinaemia and immunoblastic proliferation associated with a selective defect in immune interferon secretion. Lancet. 1978 Jul 29;2(8083):231–234. doi: 10.1016/s0140-6736(78)91744-0. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Movahed L. A., Warnke R. A., Sklar J. Detection of Epstein-Barr viral genomes in Reed-Sternberg cells of Hodgkin's disease. N Engl J Med. 1989 Feb 23;320(8):502–506. doi: 10.1056/NEJM198902233200806. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Dorfman R. F., Horning S. J., Warnke R. A., Sklar J. Clonal T-cell populations in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Am J Pathol. 1986 Mar;122(3):392–397. [PMC free article] [PubMed] [Google Scholar]
- Wolf H., Haus M., Wilmes E. Persistence of Epstein-Barr virus in the parotid gland. J Virol. 1984 Sep;51(3):795–798. doi: 10.1128/jvi.51.3.795-798.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]