Abstract
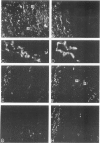
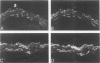
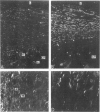
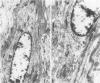
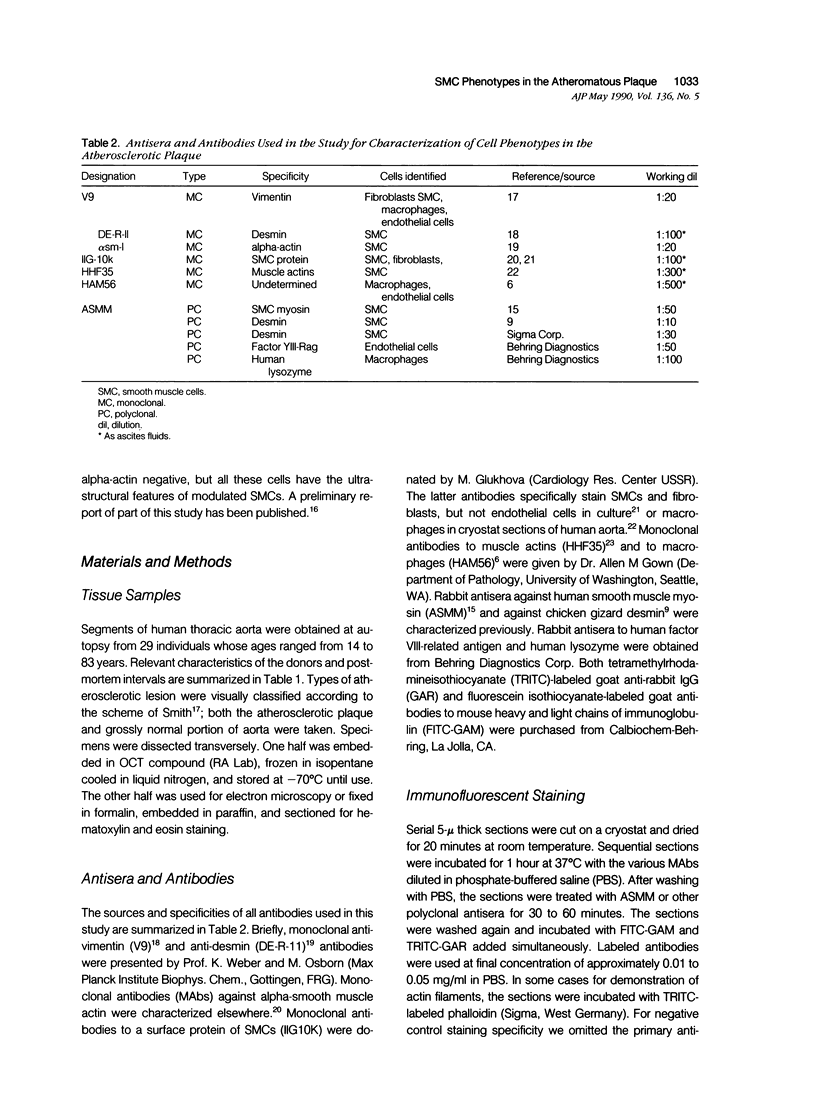
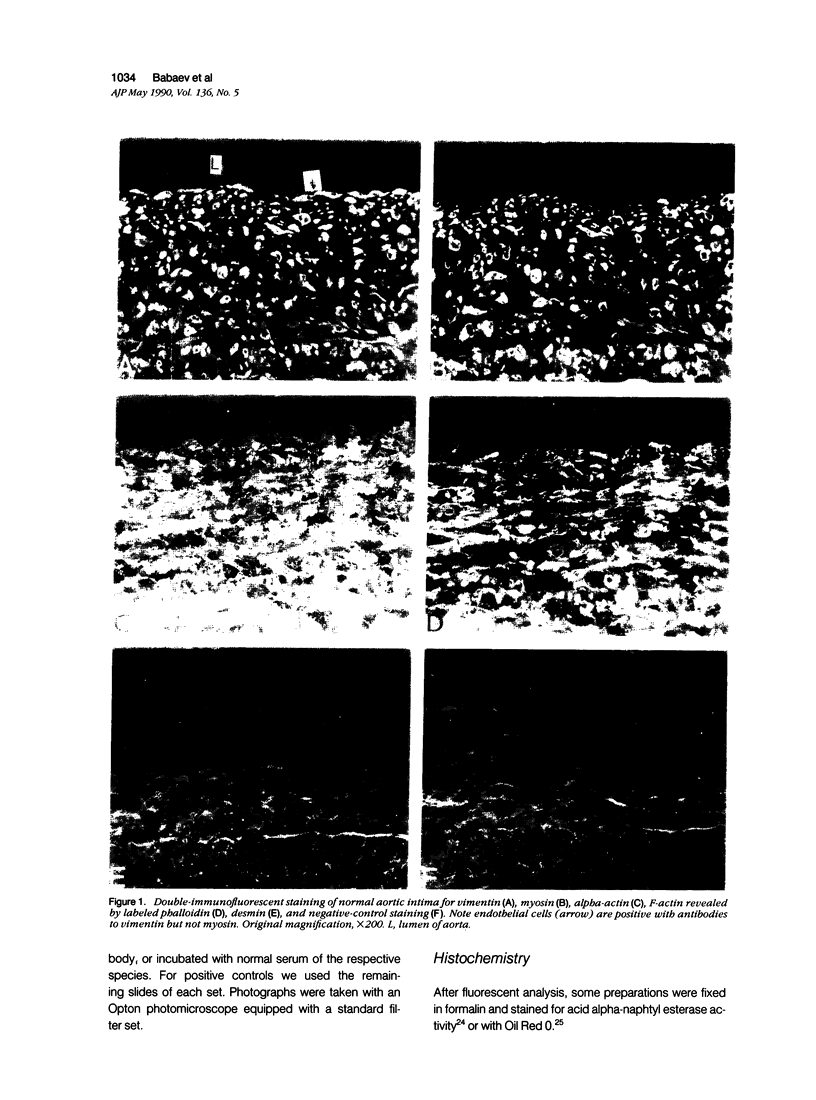
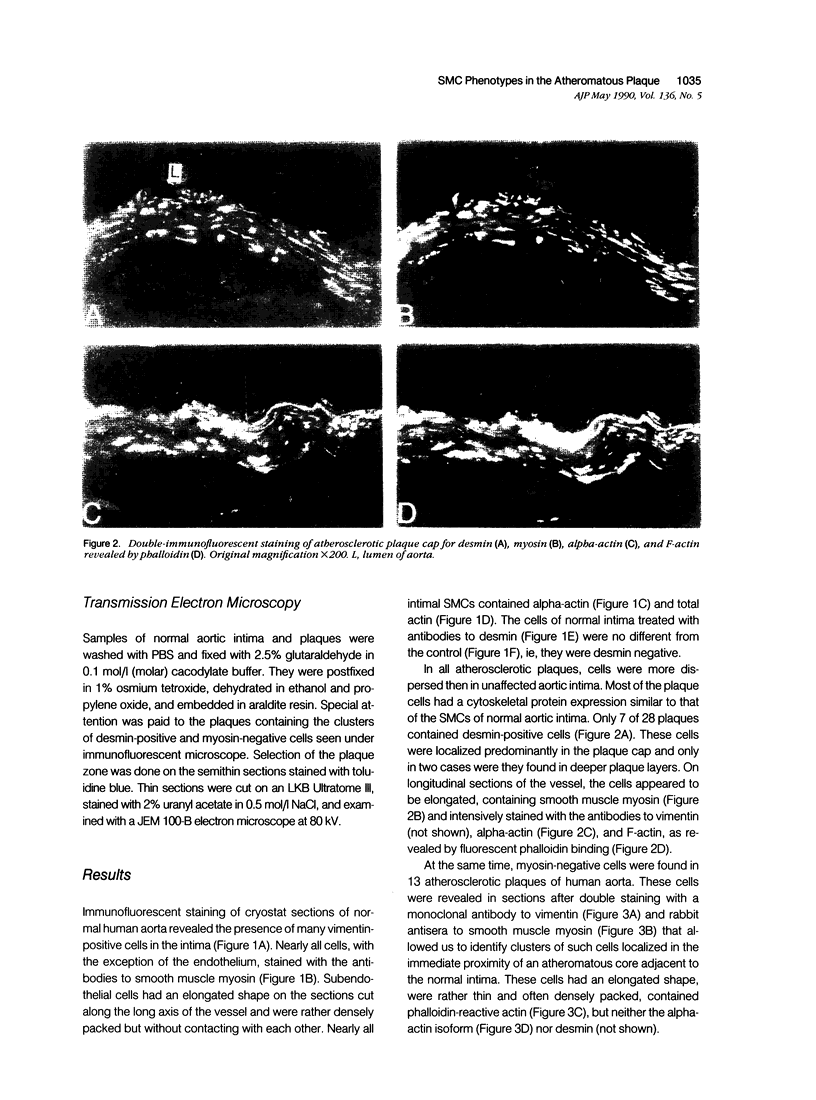
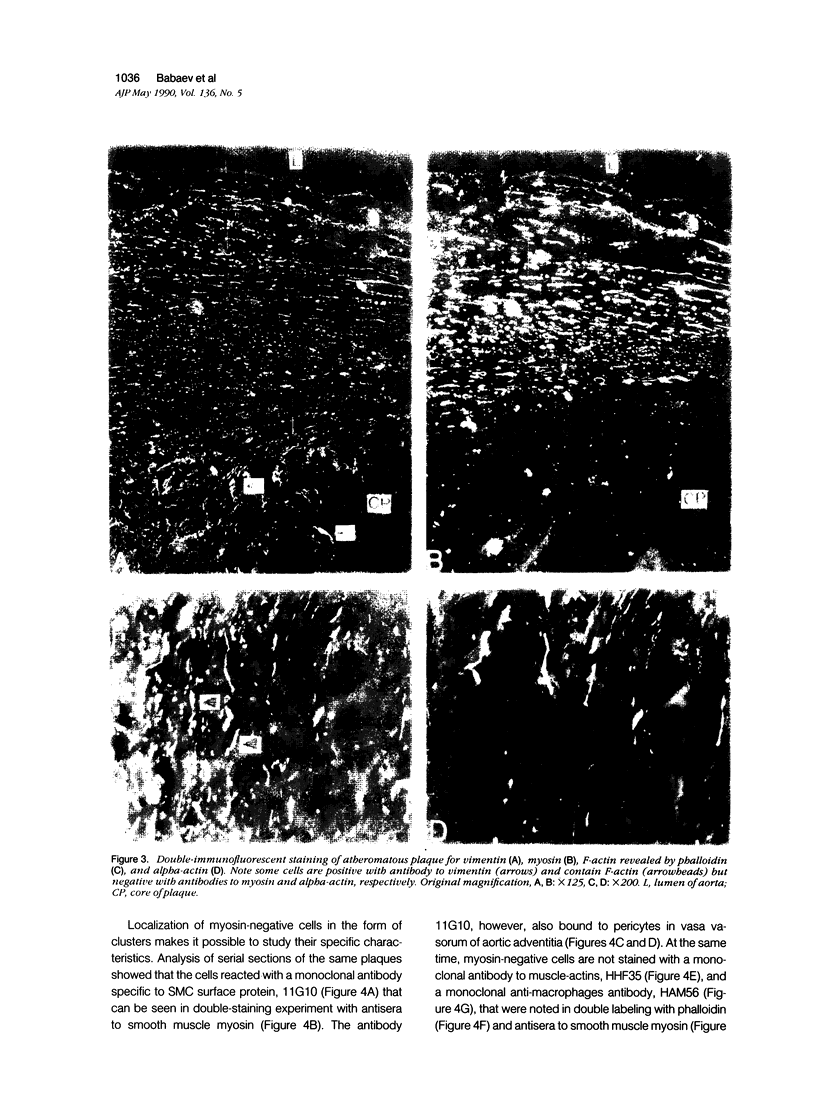
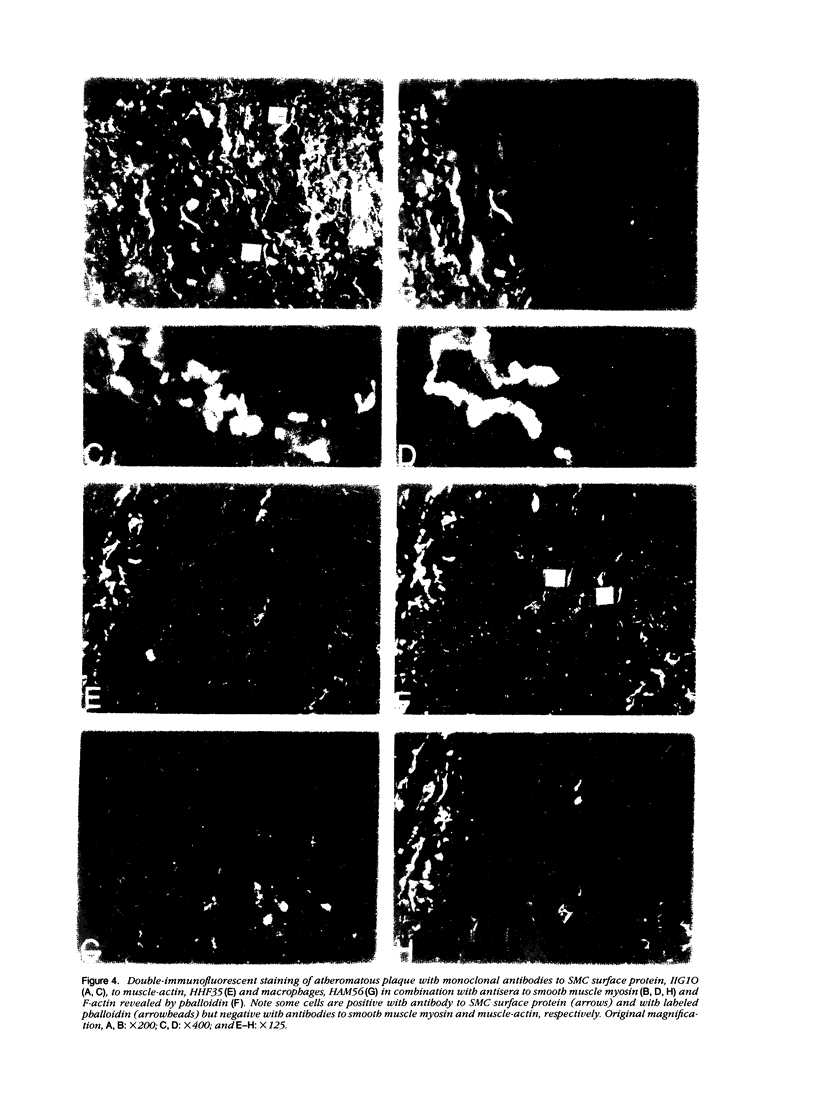
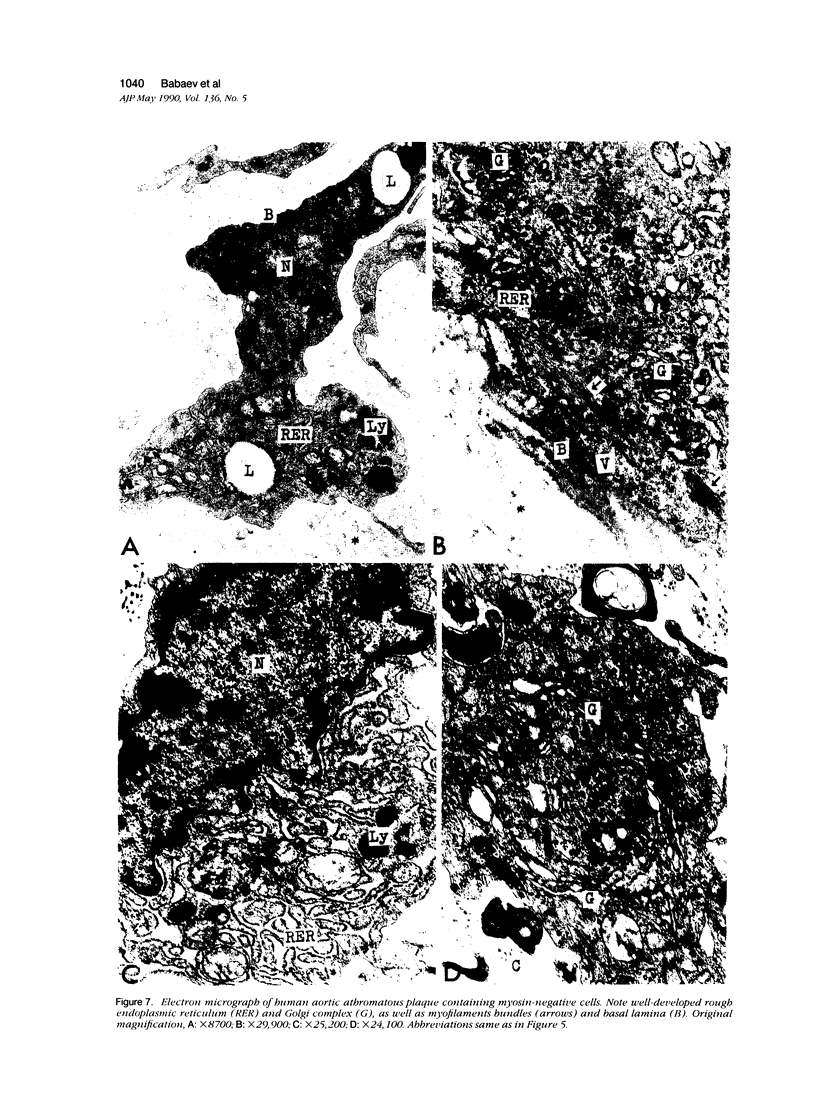
This study was undertaken to investigate the expression of cytoskeletal proteins and the ultrastructure of cells in normal intima and atheromatous plaque of human aorta. It has been established, using double-labeling immunofluorescence, that smooth muscle cells (SMC) in normal aortic intima contain myosin, vimentin, and alpha-actin but do not react with antibodies against desmin. In contrast, 7 of 28 atherosclerotic plaques contained many cells expressing desmin in addition to the other cytoskeletal proteins characteristic of normal intima SMC. These cells were localized predominantly in the plaque cap and had the ultrastructural features of modulated SMC, ie, well-developed endoplasmic reticulum and Golgi apparatus. Besides, some cells in the 13 atherosclerotic plaques proved to be myosin, alpha actin, and desmin negative but contained vimentin and actin as revealed by fluorescent phalloidin. These cells were found in the immediate proximity of atheromatous material and reacted with a monoclonal antibody specific to SMC surface protein (11G10) but not with monoclonal anti-muscle actin (HHF35) and anti-macrophage (HAM56) antibodies. Electron microscopy of this plaque zone revealed that the cytoplasm of these cells was filled with rough endoplasmic reticulum and a developed Golgi complex. At the same time, a certain proportion of cells in this region retained morphologic features of differentiated SMC such as the presence of a basal lamina and myofilament bundles. The revealed peculiarities of cytoskeletal protein expression and the ultrastructure of cells in human aortic atherosclerotic plaques may be explained by a phenotypic modulation of vascular SMC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babaev V. R., Antonov A. S., Zacharova O. S., Romanov Y. A., Krushinsky A. V., Tsibulsky V. P., Shirinsky V. P., Repin V. S., Smirnov V. N. Identification of intimal subendothelial cells from human aorta in primary culture. Atherosclerosis. 1988 May;71(1):45–56. doi: 10.1016/0021-9150(88)90301-2. [DOI] [PubMed] [Google Scholar]
- Campbell G. R., Campbell J. H. Smooth muscle phenotypic changes in arterial wall homeostasis: implications for the pathogenesis of atherosclerosis. Exp Mol Pathol. 1985 Apr;42(2):139–162. doi: 10.1016/0014-4800(85)90023-1. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. doi: 10.1002/j.1460-2075.1983.tb01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennist D. L., Jones K. H. Rapid method for identification of macrophages in suspension by acid alpha-naphthyl acetate esterase activity. J Histochem Cytochem. 1983 Jul;31(7):960–963. doi: 10.1177/31.7.6189884. [DOI] [PubMed] [Google Scholar]
- Fatigati V., Murphy R. A. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem. 1984 Dec 10;259(23):14383–14388. [PubMed] [Google Scholar]
- Frank E. D., Warren L. Aortic smooth muscle cells contain vimentin instead of desmin. Proc Natl Acad Sci U S A. 1981 May;78(5):3020–3024. doi: 10.1073/pnas.78.5.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Schmid E., Winter S., Chaponnier C., de Ckhastonay C., Vandekerckhove J., Weber K., Franke W. W. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981 Jan;78(1):298–302. doi: 10.1073/pnas.78.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Kabakov A. Y., Ornatsky O. I., Frid M. G., Smirnov V. N. Monoclonal antibodies that distinguish between human aorta smooth muscle and endothelial cells. FEBS Lett. 1985 Sep 23;189(2):291–295. doi: 10.1016/0014-5793(85)81042-5. [DOI] [PubMed] [Google Scholar]
- Glukhova M. A., Ornatsky O. I., Frid M. G., Kabakov A. E., Adany R. R., Muszbek L., Smirnov V. N. Identification of smooth muscle-derived foam cells in the atherosclerotic plaque of human aorta with monoclonal antibody IIG10. Tissue Cell. 1987;19(5):657–663. doi: 10.1016/0040-8166(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hedin U., Bottger B. A., Forsberg E., Johansson S., Thyberg J. Diverse effects of fibronectin and laminin on phenotypic properties of cultured arterial smooth muscle cells. J Cell Biol. 1988 Jul;107(1):307–319. doi: 10.1083/jcb.107.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Cytoskeletal features of normal and atheromatous human arterial smooth muscle cells. Hum Pathol. 1986 Sep;17(9):875–880. doi: 10.1016/s0046-8177(86)80637-2. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Larson D. M., Fujiwara K., Alexander R. W., Gimbrone M. A., Jr Myosin in cultured vascular smooth muscle cells: immunofluorescence and immunochemical studies of alterations in antigenic expression. J Cell Biol. 1984 Nov;99(5):1582–1589. doi: 10.1083/jcb.99.5.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosse P. R., Campbell G. R., Wang Z. L., Campbell J. H. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985 Nov;53(5):556–562. [PubMed] [Google Scholar]
- Nilsson J. Growth factors and the pathogenesis of atherosclerosis. Atherosclerosis. 1986 Dec;62(3):185–199. doi: 10.1016/0021-9150(86)90093-6. [DOI] [PubMed] [Google Scholar]
- Orekhov A. N., Kalantarov G. F., Andreeva E. R., Prokazova N. V., Trakht I. N., Bergelson L. D., Smirnov V. N. Monoclonal antibody reveals heterogeneity in human aortic intima: detection of a ganglioside antigen associated with a subpopulation of intimal cells. Am J Pathol. 1986 Mar;122(3):379–385. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Caselitz J., Püschel K., Weber K. Intermediate filament expression in human vascular smooth muscle and in arteriosclerotic plaques. Virchows Arch A Pathol Anat Histopathol. 1987;411(5):449–458. doi: 10.1007/BF00735226. [DOI] [PubMed] [Google Scholar]
- Osborn M., Debus E., Weber K. Monoclonal antibodies specific for vimentin. Eur J Cell Biol. 1984 May;34(1):137–143. [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennick R. E., Campbell J. H., Campbell G. R. Vascular smooth muscle phenotype and growth behaviour can be influenced by macrophages in vitro. Atherosclerosis. 1988 May;71(1):35–43. doi: 10.1016/0021-9150(88)90300-0. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Rovner A. S., Murphy R. A., Owens G. K. Expression of smooth muscle and nonmuscle myosin heavy chains in cultured vascular smooth muscle cells. J Biol Chem. 1986 Nov 5;261(31):14740–14745. [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Nilsson J., Palmberg L., Sjölund M. Adult human arterial smooth muscle cells in primary culture. Modulation from contractile to synthetic phenotype. Cell Tissue Res. 1985;239(1):69–74. doi: 10.1007/BF00214904. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]