Abstract
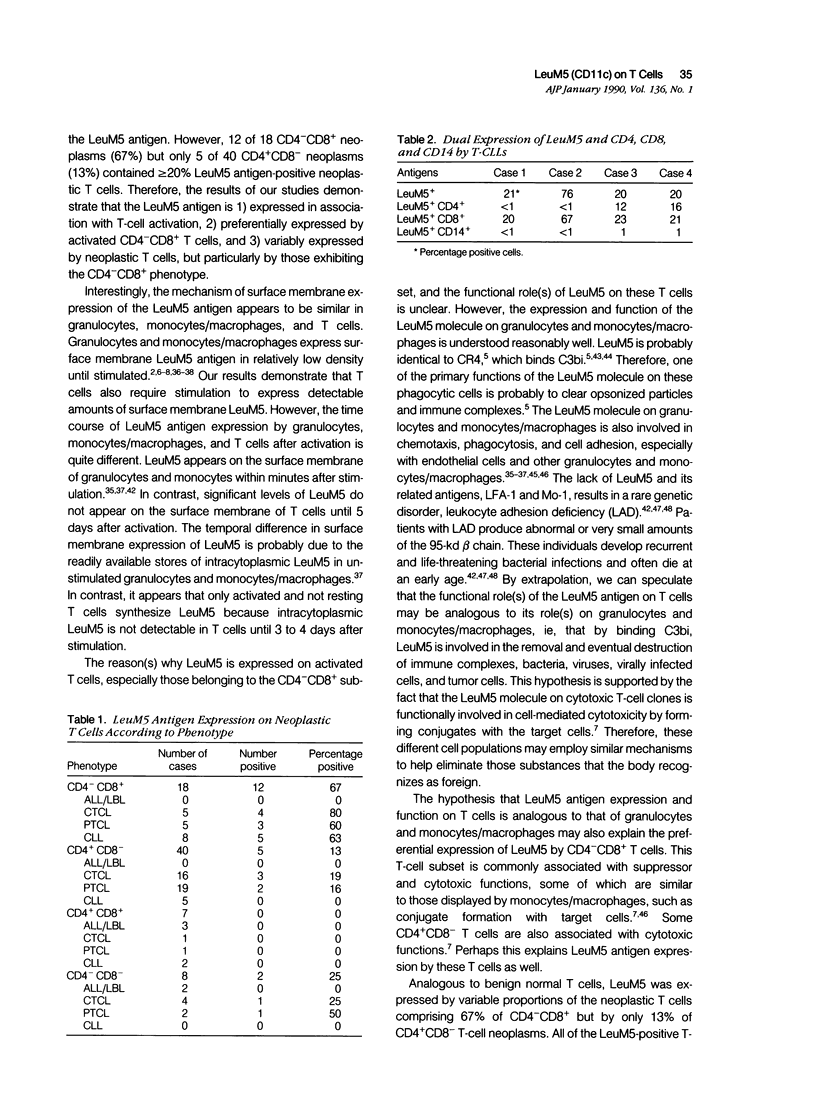
LeuM5 antigen (CD11c, p150.95) expression, widely used as an immunodiagnostic marker for B-cell hairy cell leukemia, was examined on benign, normal peripheral blood T cells before and after stimulation in vitro with phytohemagglutinin and on a large, diverse panel of 73 T-cell neoplasms. Resting T cells lacked LeuM5. Intracytoplasmic LeuM5 was detectable at 3 to 4 days and surface membrane LeuM5 was detectable continuously between 5 and 17 days on greater than or equal to 20% CD3 cells (maximum, 42% CD3 cells at 10 days) after activation. Two-color flow cytometric analysis of the activated T cells demonstrated that a maximum of 60% CD8 but only 25% CD4 cells expressed LeuM5; the mean percentage of LeuM5+ CD8 cells was 44% compared with 12% LeuM5+ CD4 cells. A variable proportion of the neoplastic T cells in 19 of 73 (26%) T-cell neoplasms were LeuM5+. Twelve of 18 CD4-CD8+ (67%) but only 5 of 40 CD4+ CD8- T-cell neoplasms expressed LeuM5. These studies demonstrate that the LeuM5 antigen is 1) expressed in association with T-cell activation, 2) preferentially expressed by activated CD8 cells, and 3) variably expressed by neoplastic T cells, but particularly by those exhibiting the CD4- CD8+ phenotype.
Full text
PDF


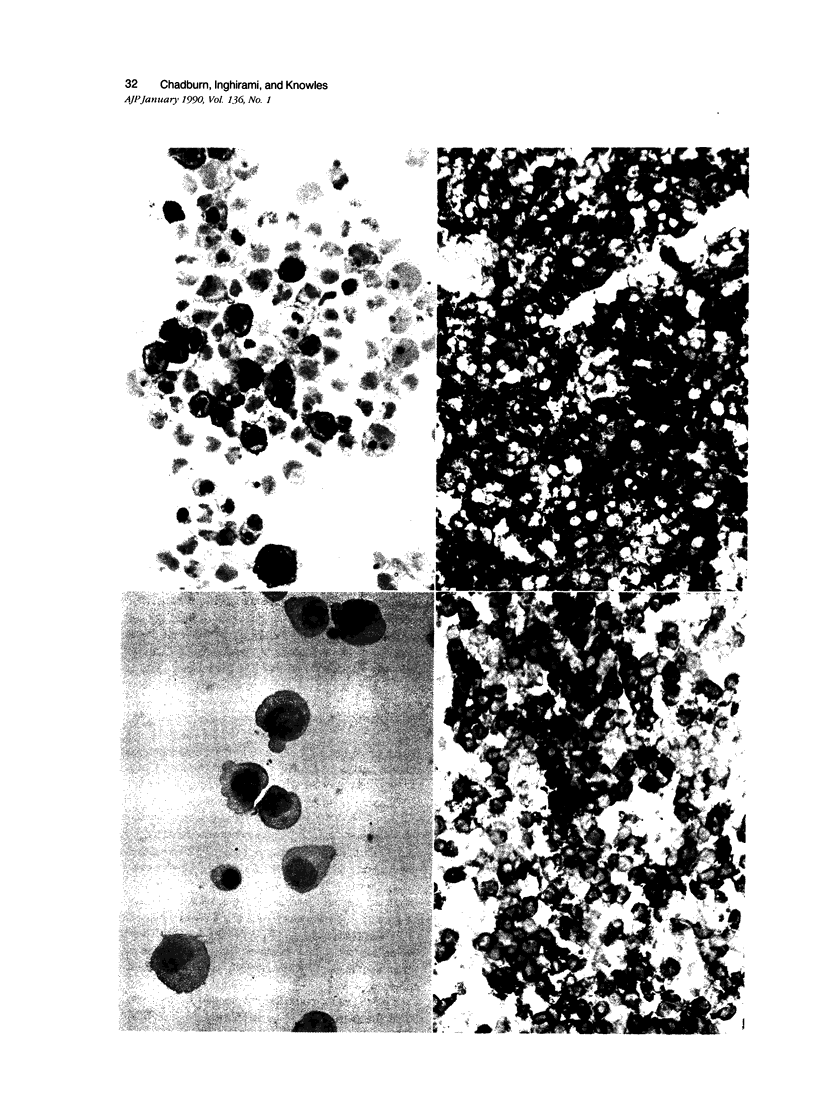
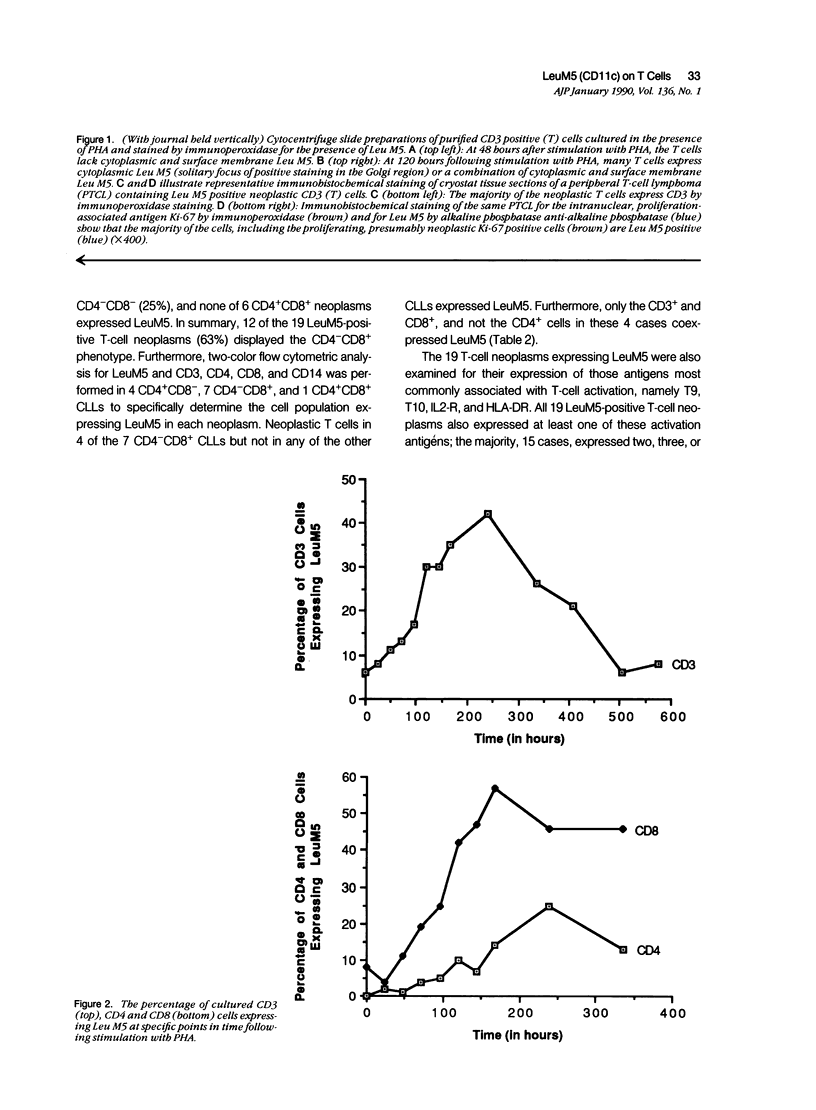
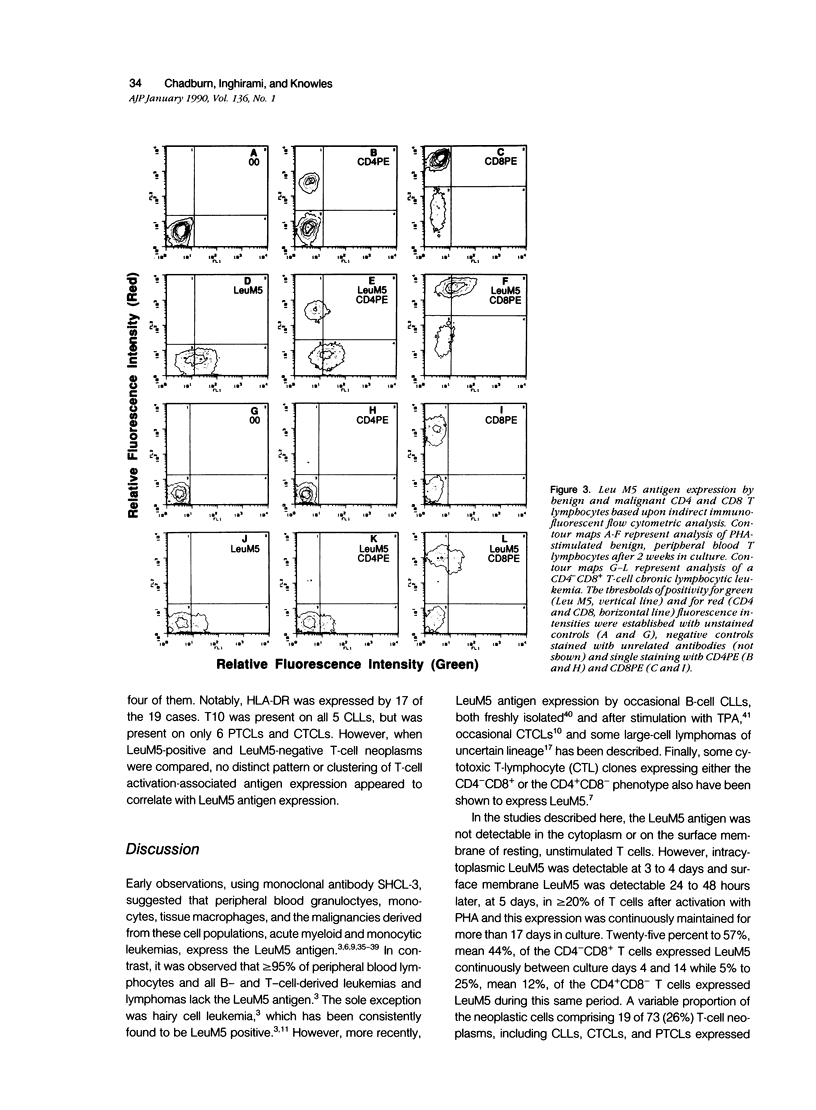


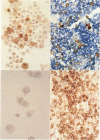
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnarsson B. A., Kadin M. E. Ki-1 positive large cell lymphoma. A morphologic and immunologic study of 19 cases. Am J Surg Pathol. 1988 Apr;12(4):264–274. doi: 10.1097/00000478-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Al Saati T., Caveriviere P., Gorguet B., Delsol G., Gatter K. C., Mason D. Y. Epithelial membrane antigen in hematopoietic neoplasms. Hum Pathol. 1986 May;17(5):533–534. doi: 10.1016/s0046-8177(86)80046-6. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Andreesen R., Osterholz J., Löhr G. W., Bross K. J. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984 Jun;63(6):1299–1302. [PubMed] [Google Scholar]
- Arnaout M. A., Lanier L. L., Faller D. V. Relative contribution of the leukocyte molecules Mo1, LFA-1, and p150,95 (LeuM5) in adhesion of granulocytes and monocytes to vascular endothelium is tissue- and stimulus-specific. J Cell Physiol. 1988 Nov;137(2):305–309. doi: 10.1002/jcp.1041370214. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Wang E. A., Clark S. C., Sieff C. A. Human recombinant granulocyte-macrophage colony-stimulating factor increases cell-to-cell adhesion and surface expression of adhesion-promoting surface glycoproteins on mature granulocytes. J Clin Invest. 1986 Aug;78(2):597–601. doi: 10.1172/JCI112615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabañas C., Sanchez-Madrid F., Acevedo A., Bellon T., Fernandez J. M., Larraga V., Bernabeu C. Characterization of a CD11c-reactive monoclonal antibody (HC1/1) obtained by immunizing with phorbol ester differentiated U937 cells. Hybridoma. 1988 Apr;7(2):167–176. doi: 10.1089/hyb.1988.7.167. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Pizzolo G., Chilosi M., Bergui L., Semenzato G., Tesio L., Morittu L., Malavasi F., Gobbi M., Schwarting R. Phorbol ester induces abnormal chronic lymphocytic leukemia cells to express features of hairy cell leukemia. Blood. 1985 Nov;66(5):1035–1042. [PubMed] [Google Scholar]
- Chilosi M., Menestrina F., Capelli P., Montagna L., Lestani M., Pizzolo G., Cipriani A., Agostini C., Trentin L., Zambello R. Immunohistochemical analysis of sarcoid granulomas. Evaluation of Ki67+ and interleukin-1+ cells. Am J Pathol. 1988 May;131(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- De la Hera A., Alvarez-Mon M., Sanchez-Madrid F., Martinez C., Durantez A. Co-expression of Mac-1 and p150,95 on CD5+ B cells. Structural and functional characterization in a human chronic lymphocytic leukemia. Eur J Immunol. 1988 Jul;18(7):1131–1134. doi: 10.1002/eji.1830180725. [DOI] [PubMed] [Google Scholar]
- Delsol G., Al Saati T., Gatter K. C., Gerdes J., Schwarting R., Caveriviere P., Rigal-Huguet F., Robert A., Stein H., Mason D. Y. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988 Jan;130(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Delsol G., Gatter K. C., Stein H., Erber W. N., Pulford K. A., Zinne K., Mason D. Y. Human lymphoid cells express epithelial membrane antigen. Implications for diagnosis of human neoplasms. Lancet. 1984 Nov 17;2(8412):1124–1129. doi: 10.1016/s0140-6736(84)91558-7. [DOI] [PubMed] [Google Scholar]
- Falini B., Pulford K., Erber W. N., Posnett D. N., Pallesen G., Schwarting R., Annino L., Cafolla A., Canino S., Mori A. Use of a panel of monoclonal antibodies for the diagnosis of hairy cell leukaemia. An immunocytochemical study of 36 cases. Histopathology. 1986 Jul;10(7):671–687. doi: 10.1111/j.1365-2559.1986.tb02521.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwarting R., Stein H. High proliferative activity of Reed Sternberg associated antigen Ki-1 positive cells in normal lymphoid tissue. J Clin Pathol. 1986 Sep;39(9):993–997. doi: 10.1136/jcp.39.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorno R. Immunohistochemical analysis of human peripheral blood and lymphoid tissues using monoclonal antibodies immunoreactive with non-lymphoid cells. Histochemistry. 1986;84(3):241–245. doi: 10.1007/BF00495789. [DOI] [PubMed] [Google Scholar]
- Hanjan S. N., Kearney J. F., Cooper M. D. A monoclonal antibody (MMA) that identifies a differentiation antigen on human myelomonocytic cells. Clin Immunol Immunopathol. 1982 May;23(2):172–188. doi: 10.1016/0090-1229(82)90106-4. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Inghirami G., Wieczorek R., Zhu B. Y., Silber R., Dalla-Favera R., Knowles D. M. Differential expression of LFA-1 molecules in non-Hodgkin's lymphoma and lymphoid leukemia. Blood. 1988 Oct;72(4):1431–1434. [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Figdor C. G., Spits H., Miedema F., Terhorst C., De Vries J. E. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol. 1985 Nov;15(11):1142–1148. doi: 10.1002/eji.1830151114. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Visser W., Schwarting R., de Vries J. E., Figdor C. G. Membrane glycoprotein p150,95 of human cytotoxic T cell clone is involved in conjugate formation with target cells. J Immunol. 1987 May 15;138(10):3130–3136. [PubMed] [Google Scholar]
- Keizer G. D., Te Velde A. A., Schwarting R., Figdor C. G., De Vries J. E. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987 Sep;17(9):1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Kishimoto T. K., Hollander N., Roberts T. M., Anderson D. C., Springer T. A. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987 Jul 17;50(2):193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Arnaout M. A., Schwarting R., Warner N. L., Ross G. D. p150/95, Third member of the LFA-1/CR3 polypeptide family identified by anti-Leu M5 monoclonal antibody. Eur J Immunol. 1985 Jul;15(7):713–718. doi: 10.1002/eji.1830150714. [DOI] [PubMed] [Google Scholar]
- Malhotra V., Hogg N., Sim R. B. Ligand binding by the p150,95 antigen of U937 monocytic cells: properties in common with complement receptor type 3 (CR3). Eur J Immunol. 1986 Sep;16(9):1117–1123. doi: 10.1002/eji.1830160915. [DOI] [PubMed] [Google Scholar]
- Micklem K. J., Sim R. B. Isolation of complement-fragment-iC3b-binding proteins by affinity chromatography. The identification of p150,95 as an iC3b-binding protein. Biochem J. 1985 Oct 1;231(1):233–236. doi: 10.1042/bj2310233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Bainton D. F., Borregaard N., Springer T. A. Stimulated mobilization of monocyte Mac-1 and p150,95 adhesion proteins from an intracellular vesicular compartment to the cell surface. J Clin Invest. 1987 Aug;80(2):535–544. doi: 10.1172/JCI113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. J., Schwarting R., Springer T. A. Regulated expression of the Mac-1, LFA-1, p150,95 glycoprotein family during leukocyte differentiation. J Immunol. 1986 Nov 1;137(9):2891–2900. [PubMed] [Google Scholar]
- Miller L. J., Springer T. A. Biosynthesis and glycosylation of p150,95 and related leukocyte adhesion proteins. J Immunol. 1987 Aug 1;139(3):842–847. [PubMed] [Google Scholar]
- Myones B. L., Dalzell J. G., Hogg N., Ross G. D. Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest. 1988 Aug;82(2):640–651. doi: 10.1172/JCI113643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus G. S., Kurtin P. J. Epithelial membrane antigen--a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol. 1985 Sep;16(9):929–940. doi: 10.1016/s0046-8177(85)80132-5. [DOI] [PubMed] [Google Scholar]
- Pinkus G. S., Thomas P., Said J. W. Leu-M1--a marker for Reed-Sternberg cells in Hodgkin's disease. An immunoperoxidase study of paraffin-embedded tissues. Am J Pathol. 1985 May;119(2):244–252. [PMC free article] [PubMed] [Google Scholar]
- Salhany K. E., Cousar J. B., Greer J. P., Casey T. T., Fields J. P., Collins R. D. Transformation of cutaneous T cell lymphoma to large cell lymphoma. A clinicopathologic and immunologic study. Am J Pathol. 1988 Aug;132(2):265–277. [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Springer T. A., Miller L. J., Anderson D. C. p150,95, the third member of the Mac-1, LFA-1 human leukocyte adhesion glycoprotein family. J Immunol. 1986 Jan;136(1):240–245. [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Swerdlow S. H., Wright S. A. The spectrum of Leu-M1 staining in lymphoid and hematopoietic proliferations. Am J Clin Pathol. 1986 Mar;85(3):283–288. doi: 10.1093/ajcp/85.3.283. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Picker L. J., Copenhaver C. M., Warnke R. A., Sklar J. Large-cell hematolymphoid neoplasms of uncertain lineage. Hum Pathol. 1988 Aug;19(8):967–973. doi: 10.1016/s0046-8177(88)80014-5. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Strickler J. G., Medeiros L. J., Gerdes J., Stein H., Warnke R. A. Proliferative rates of non-Hodgkin's lymphomas as assessed by Ki-67 antibody. Hum Pathol. 1987 Nov;18(11):1155–1159. doi: 10.1016/s0046-8177(87)80384-2. [DOI] [PubMed] [Google Scholar]
- Wieczorek R., Burke J. S., Knowles D. M., 2nd Leu-M1 antigen expression in T-cell neoplasia. Am J Pathol. 1985 Dec;121(3):374–380. [PMC free article] [PubMed] [Google Scholar]
- Wieczorek R., Suhrland M., Ramsay D., Reed M. L., Knowles D. M., 2nd Leu-M1 antigen expression in advanced (tumor) stage mycosis fungoides. Am J Clin Pathol. 1986 Jul;86(1):25–32. doi: 10.1093/ajcp/86.1.25. [DOI] [PubMed] [Google Scholar]
- te Velde A. A., Keizer G. D., Figdor C. G. Differential function of LFA-1 family molecules (CD11 and CD18) in adhesion of human monocytes to melanoma and endothelial cells. Immunology. 1987 Jul;61(3):261–267. [PMC free article] [PubMed] [Google Scholar]