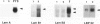Abstract
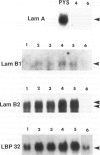
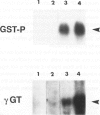
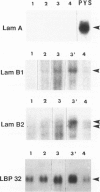
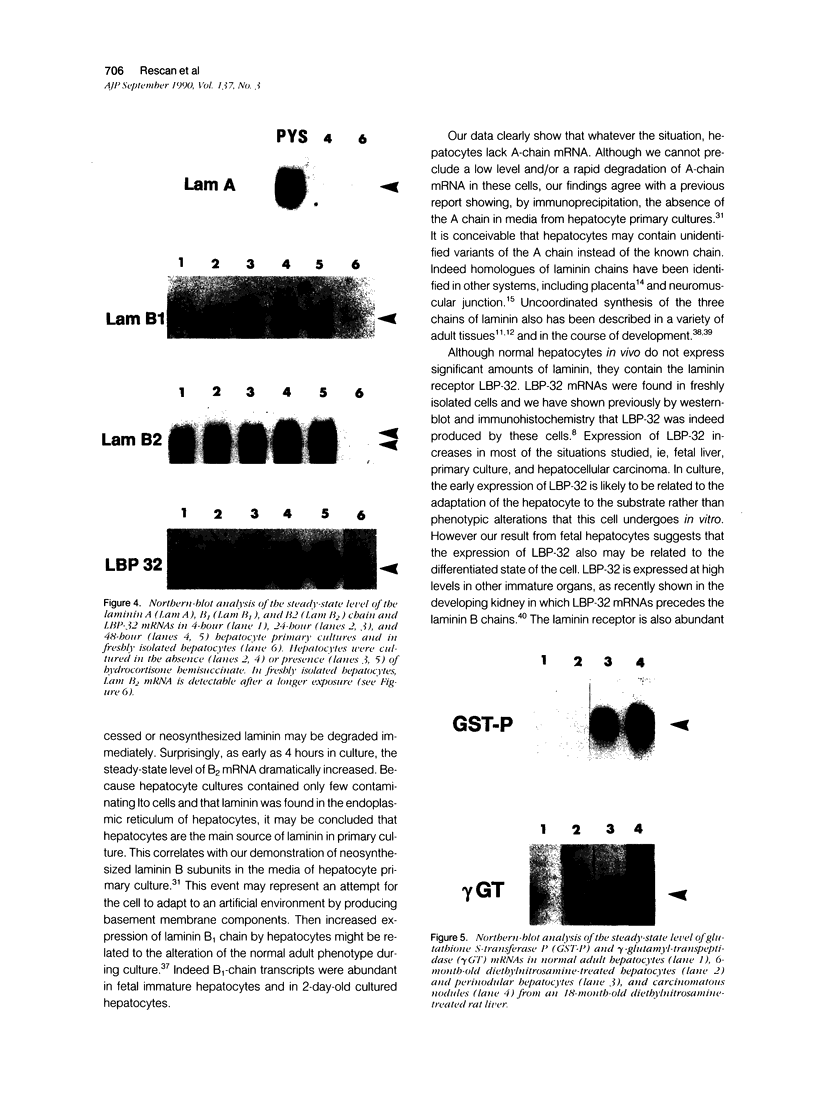
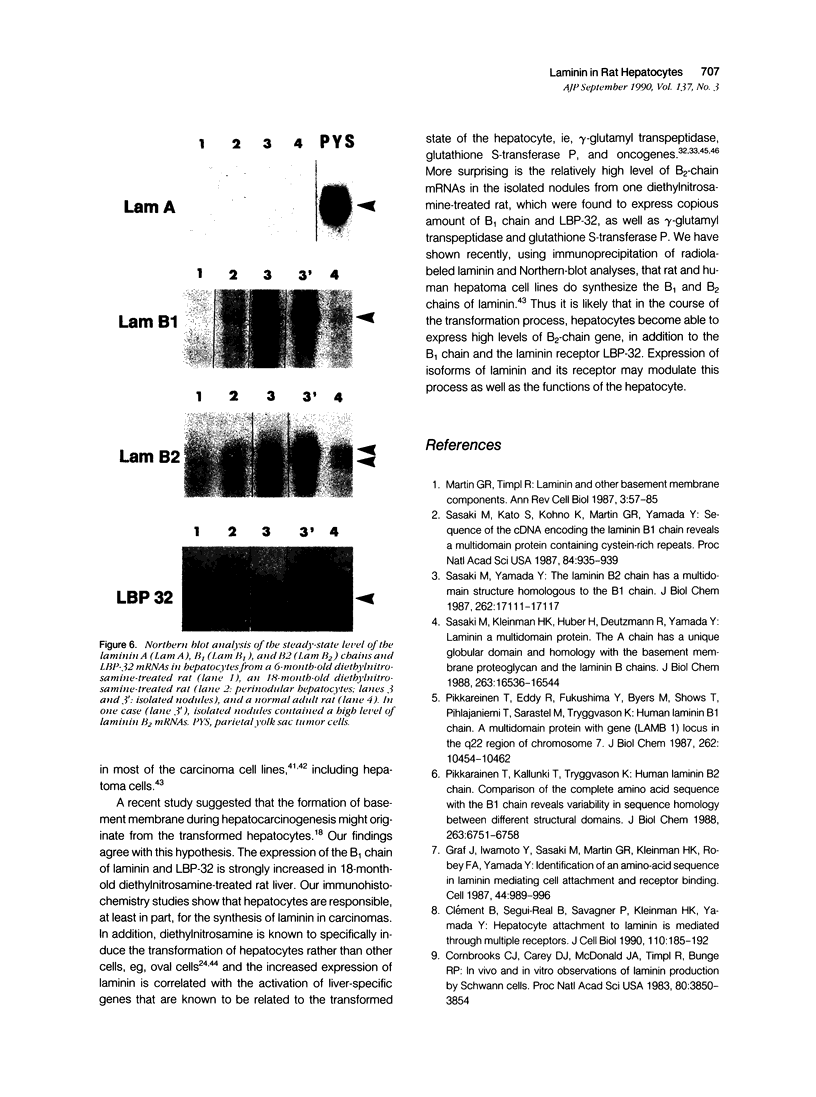
Laminin deposition is increased in fetal liver and in a variety of liver diseases, including the development of carcinoma. We investigated the role of the hepatocyte in the synthesis of both laminin and its 32- to 67-kd receptor in normal adult, fetal, and diethylnitrosamine-treated rat livers, and in adult rat hepatocyte primary cultures. Laminin was localized by immunoelectron microscopy in the endoplasmic reticulum of hepatocytes in fetal-derived and in 18-month-old diethylnitrosamine-treated rat livers. Steady-state mRNA levels for the three chains of laminin (A, B1, and B2) and the laminin receptor (LBP-32) were examined. Northern-blot analyses showed that hepatocytes at all stages lacked the A-chain mRNA. B1-chain mRNA was undetectable in normal adult hepatocytes, while significant levels of B1-chain mRNA were found in fetal hepatocytes and in adult hepatocyte primary culture. In hepatocytes from diethylnitrosamine-treated rats, B1-chain mRNAs were abundant and were present mainly in nodular formations rather than in the nontumorous areas. B2-chain mRNAs were barely detectable in either normal adult or fetal hepatocytes. In diethylnitrosamine-treated rats, the steady-state B2-chain mRNA level was higher in nodules than in nontumorous areas. In primary culture, B2-chain mRNAs were present as early as 4 hours after adult hepatocyte seeding, and dramatically increased during the following 2 days. Only low levels of laminin-receptor (LBP-32) mRNAs were present in normal adult hepatocytes, whereas the levels were high in the fetal and in the tumor-containing livers. In diethylnitrosamine-treated rats, LBP-32 mRNAs were more abundant in nodular formations rather than in nontumorous areas. In hepatocyte primary culture, the expression of the LBP-32 mRNA dramatically increased during the first 24 hours. These results show that in hepatocytes, expression of laminin chains and its receptor LBP-32 are not coordinated and depends on the maturation of the cells. In addition, they suggest that the expression of B1 and B2 chains in adult hepatocytes is related to changes of the normal phenotype and/or the pericellular environment.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Wewer U. M., Thorgeirsson S. S. De novo deposition of laminin-positive basement membrane in vitro by normal hepatocytes and during hepatocarcinogenesis. Hepatology. 1988 May-Jun;8(3):538–546. doi: 10.1002/hep.1840080318. [DOI] [PubMed] [Google Scholar]
- Aratani Y., Kitagawa Y. Enhanced synthesis and secretion of type IV collagen and entactin during adipose conversion of 3T3-L1 cells and production of unorthodox laminin complex. J Biol Chem. 1988 Nov 5;263(31):16163–16169. [PubMed] [Google Scholar]
- Bianchi F. B., Biagini G., Ballardini G., Cenacchi G., Faccani A., Pisi E., Laschi R., Liotta L., Garbisa S. Basement membrane production by hepatocytes in chronic liver disease. Hepatology. 1984 Nov-Dec;4(6):1167–1172. doi: 10.1002/hep.1840040612. [DOI] [PubMed] [Google Scholar]
- Boot-Handford R. P., Kurkinen M., Prockop D. J. Steady-state levels of mRNAs coding for the type IV collagen and laminin polypeptide chains of basement membranes exhibit marked tissue-specific stoichiometric variations in the rat. J Biol Chem. 1987 Sep 15;262(26):12475–12478. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clement B., Rissel M., Peyrol S., Mazurier Y., Grimaud J. A., Guillouzo A. A procedure for light and electron microscopic intracellular immunolocalization of collagen and fibronectin in rat liver. J Histochem Cytochem. 1985 May;33(5):407–414. doi: 10.1177/33.5.3886779. [DOI] [PubMed] [Google Scholar]
- Clément B., Rescan P. Y., Baffet G., Loréal O., Lehry D., Campion J. P., Guillouzo A. Hepatocytes may produce laminin in fibrotic liver and in primary culture. Hepatology. 1988 Jul-Aug;8(4):794–803. doi: 10.1002/hep.1840080417. [DOI] [PubMed] [Google Scholar]
- Clément B., Segui-Real B., Savagner P., Kleinman H. K., Yamada Y. Hepatocyte attachment to laminin is mediated through multiple receptors. J Cell Biol. 1990 Jan;110(1):185–192. doi: 10.1083/jcb.110.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. R., MacQueen H. A. Subunits of laminin are differentially synthesized in mouse eggs and early embryos. Dev Biol. 1983 Apr;96(2):467–471. doi: 10.1016/0012-1606(83)90183-5. [DOI] [PubMed] [Google Scholar]
- Cornbrooks C. J., Carey D. J., McDonald J. A., Timpl R., Bunge R. P. In vivo and in vitro observations on laminin production by Schwann cells. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3850–3854. doi: 10.1073/pnas.80.12.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral M., Tichonicky L., Guguen-Guillouzo C., Corcos D., Raymondjean M., Paris B., Kruh J., Defer N. Expression of c-fos oncogene during hepatocarcinogenesis, liver regeneration and in synchronized HTC cells. Exp Cell Res. 1985 Oct;160(2):427–434. doi: 10.1016/0014-4827(85)90190-9. [DOI] [PubMed] [Google Scholar]
- Graf J., Iwamoto Y., Sasaki M., Martin G. R., Kleinman H. K., Robey F. A., Yamada Y. Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis, and receptor binding. Cell. 1987 Mar 27;48(6):989–996. doi: 10.1016/0092-8674(87)90707-0. [DOI] [PubMed] [Google Scholar]
- Hunter D. D., Shah V., Merlie J. P., Sanes J. R. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989 Mar 16;338(6212):229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Klein G., Langegger M., Timpl R., Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988 Oct 21;55(2):331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ebihara I., Killen P. D., Sasaki M., Cannon F. B., Yamada Y., Martin G. R. Genes for basement membrane proteins are coordinately expressed in differentiating F9 cells but not in normal adult murine tissues. Dev Biol. 1987 Aug;122(2):373–378. doi: 10.1016/0012-1606(87)90302-2. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Barlow D. P., Jenkins J. R., Hogan B. L. In vitro synthesis of laminin and entactin polypeptides. J Biol Chem. 1983 May 25;258(10):6543–6548. [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G. W., Horikoshi S., Killen P. D., Segui-Real B., Yamada Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for a laminin receptor, laminin, and basement membrane (type IV) collagen in the developing kidney. J Cell Biol. 1989 Sep;109(3):1351–1362. doi: 10.1083/jcb.109.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., Friedman S. L., Roll F. J., Bissell D. M. Immunolocalization of laminin in normal rat liver and biosynthesis of laminin by hepatic lipocytes in primary culture. Gastroenterology. 1988 Apr;94(4):1053–1062. doi: 10.1016/0016-5085(88)90566-5. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Miller E. J., Damjanov I., Gay S. Laminin-secreting yolk sac carcinoma of the rat. Biochemical and electron immunohistochemical studies. Lab Invest. 1982 Sep;47(3):247–257. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Riecken E. O., Stein H. Cellular localization of laminin gene transcripts in normal and fibrotic human liver. Am J Pathol. 1989 Jun;134(6):1175–1182. [PMC free article] [PubMed] [Google Scholar]
- Ohno M., Martinez-Hernandez A., Ohno N., Kefalides N. A. Isolation of laminin from human placental basement membranes: amnion, chorion and chorionic microvessels. Biochem Biophys Res Commun. 1983 May 16;112(3):1091–1098. doi: 10.1016/0006-291x(83)91730-8. [DOI] [PubMed] [Google Scholar]
- Pemble S. E., Taylor J. B., Ketterer B. Tissue distribution of rat glutathione transferase subunit 7, a hepatoma marker. Biochem J. 1986 Dec 15;240(3):885–889. doi: 10.1042/bj2400885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikkarainen T., Eddy R., Fukushima Y., Byers M., Shows T., Pihlajaniemi T., Saraste M., Tryggvason K. Human laminin B1 chain. A multidomain protein with gene (LAMB1) locus in the q22 region of chromosome 7. J Biol Chem. 1987 Aug 5;262(22):10454–10462. [PubMed] [Google Scholar]
- Pikkarainen T., Kallunki T., Tryggvason K. Human laminin B2 chain. Comparison of the complete amino acid sequence with the B1 chain reveals variability in sequence homology between different structural domains. J Biol Chem. 1988 May 15;263(14):6751–6758. [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. The stages of initiation and promotion in hepatocarcinogenesis. Biochim Biophys Acta. 1980 May 6;605(2):191–215. doi: 10.1016/0304-419x(80)90004-9. [DOI] [PubMed] [Google Scholar]
- Rescan P. Y., Clément B., Grimaud J. A., Guillois B., Strain A., Guillouzo A. Participation of hepatocytes in the production of basement membrane components in human and rat liver during the perinatal period. Cell Differ Dev. 1989 Mar;26(2):131–144. doi: 10.1016/0922-3371(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Kato S., Kohno K., Martin G. R., Yamada Y. Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci U S A. 1987 Feb;84(4):935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Sasaki M., Yamada Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J Biol Chem. 1987 Dec 15;262(35):17111–17117. [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Squire R. A., Levitt M. H. Report of a workshop on classification of specific hepatocellular lesions in rats. Cancer Res. 1975 Nov;35(11 Pt 1):3214–3223. [PubMed] [Google Scholar]
- Tougard C., Louvard D., Picart R., Tixier-Vidal A. Immunocytochemical localization of laminin in rat anterior pituitary cells in vivo and in vitro. In Vitro Cell Dev Biol. 1985 Jan;21(1):57–61. doi: 10.1007/BF02620915. [DOI] [PubMed] [Google Scholar]
- Wan Y. J., Wu T. C., Chung A. E., Damjanov I. Monoclonal antibodies to laminin reveal the heterogeneity of basement membranes in the developing and adult mouse tissues. J Cell Biol. 1984 Mar;98(3):971–979. doi: 10.1083/jcb.98.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C., Sparkes R. S., Bertolotti R. Expression of differentiated functions in hepatoma cell hybrids: IX extinction and reexpression of liver-specific enzymes in rat hepatoma-Chinese hamster fibroblast hybrids. Somatic Cell Genet. 1975 Jan;1(1):27–40. doi: 10.1007/BF01538730. [DOI] [PubMed] [Google Scholar]
- Wewer U. M., Liotta L. A., Jaye M., Ricca G. A., Drohan W. N., Claysmith A. P., Rao C. N., Wirth P., Coligan J. E., Albrechtsen R. Altered levels of laminin receptor mRNA in various human carcinoma cells that have different abilities to bind laminin. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7137–7141. doi: 10.1073/pnas.83.19.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yow H. K., Wong J. M., Chen H. S., Lee C. G., Davis S., Steele G. D., Jr, Chen L. B. Increased mRNA expression of a laminin-binding protein in human colon carcinoma: complete sequence of a full-length cDNA encoding the protein. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6394–6398. doi: 10.1073/pnas.85.17.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]