Abstract
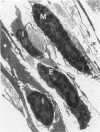
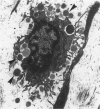
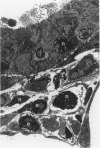
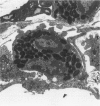
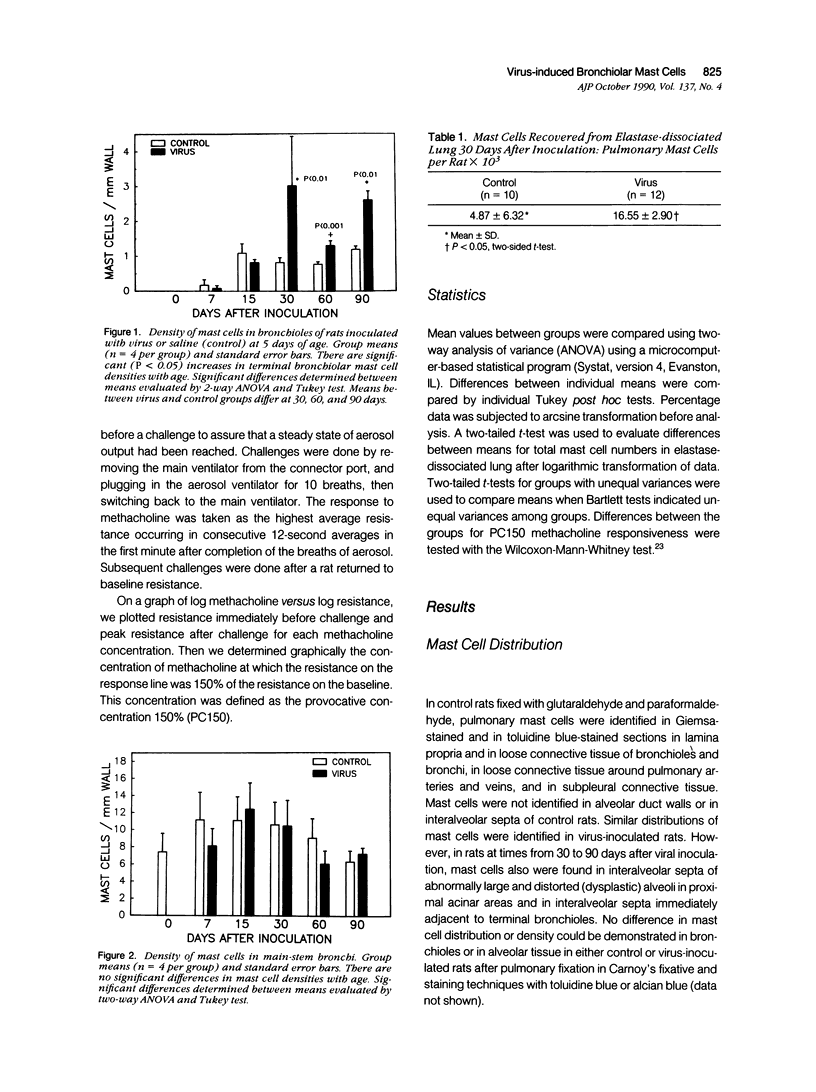
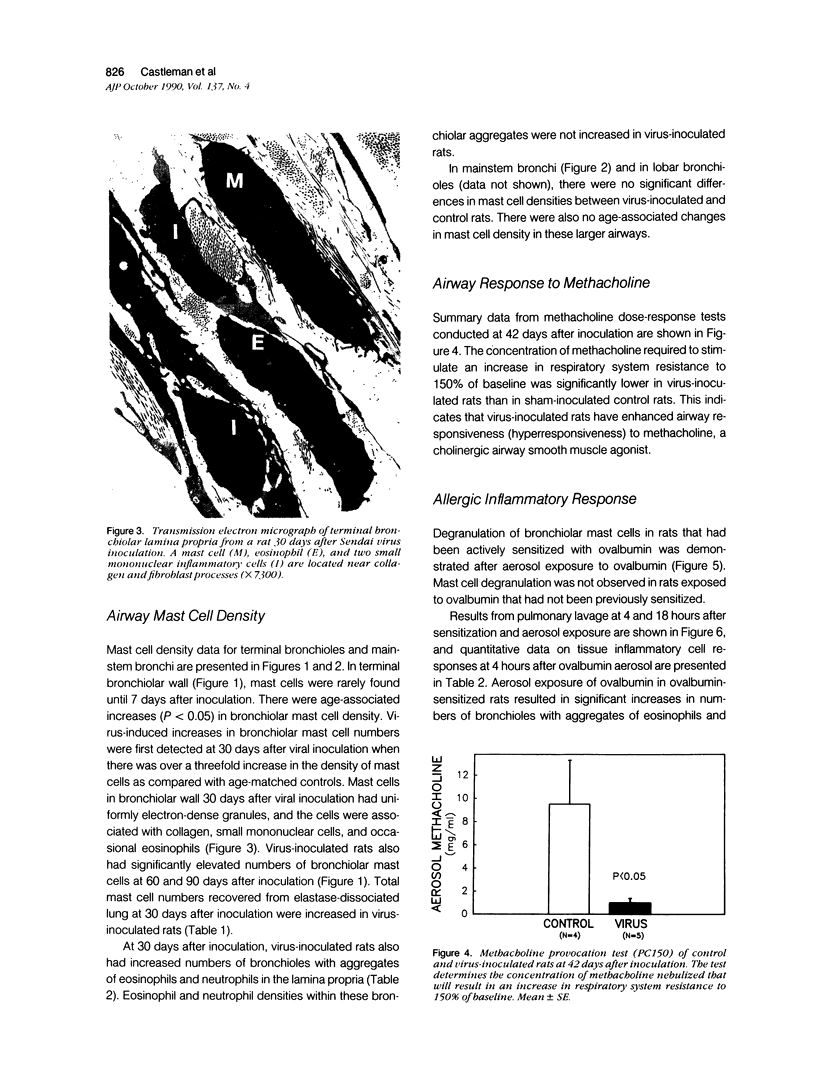
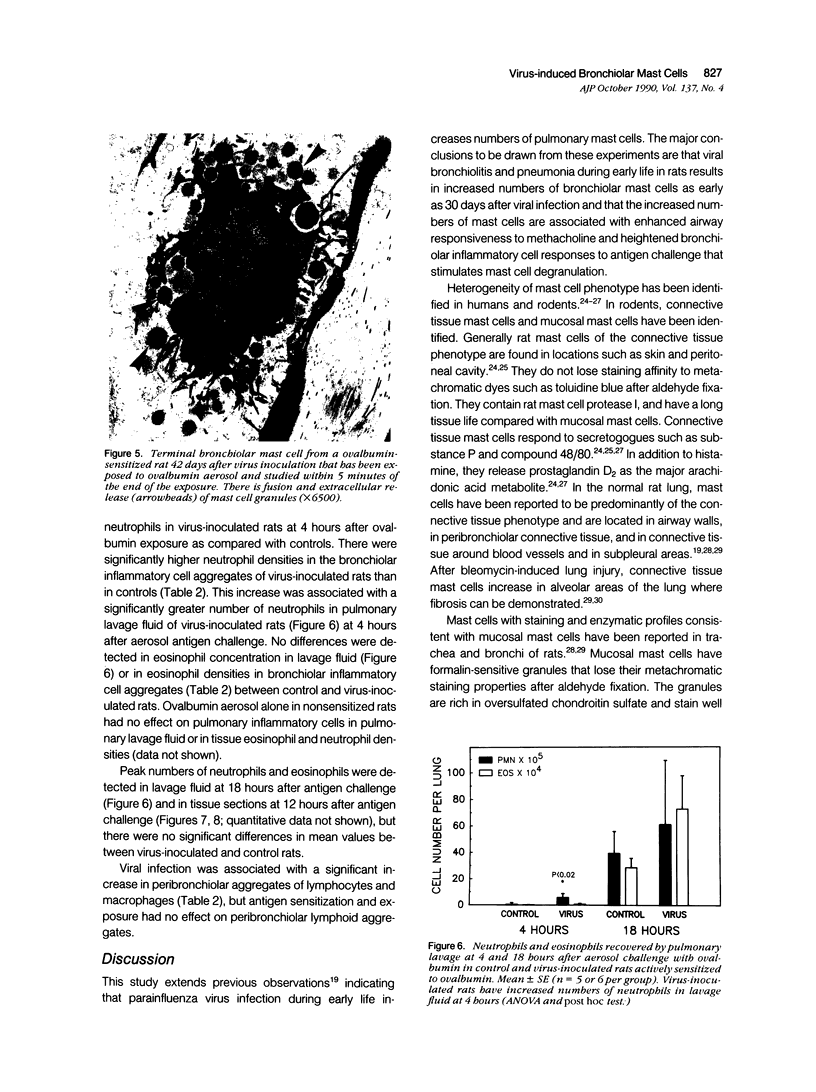
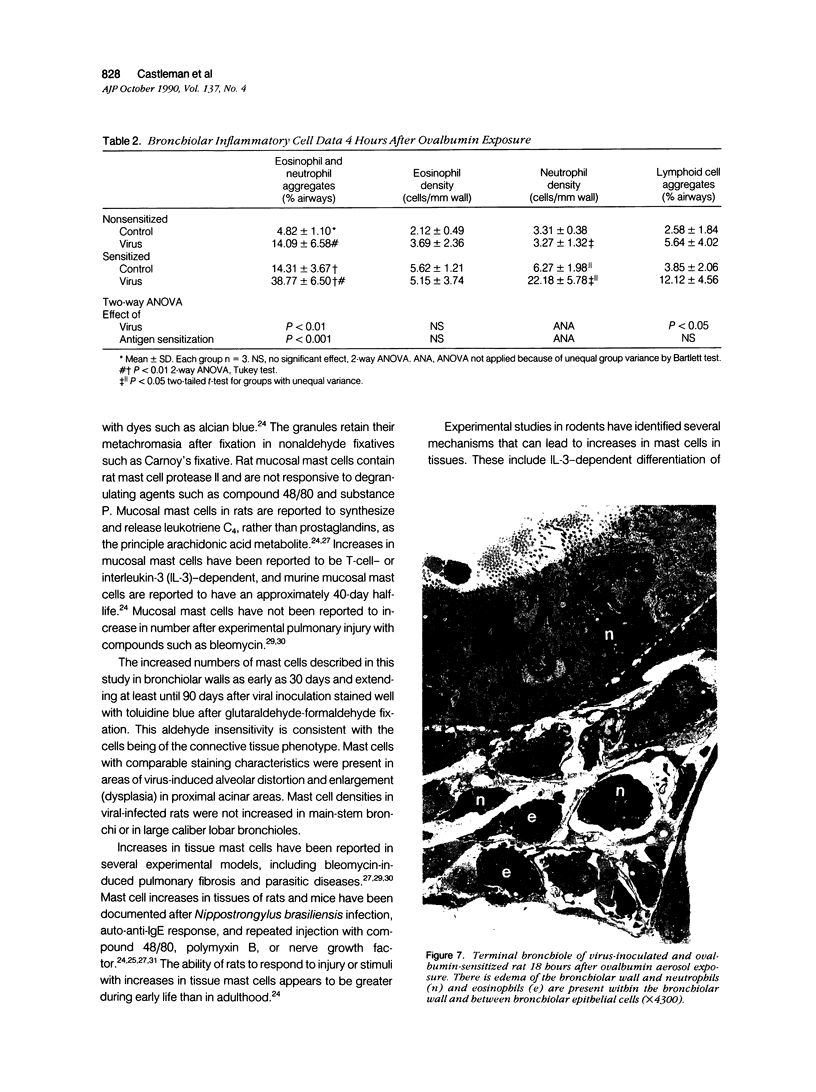
The objectives of this study were to determine the kinetics of Sendai virus-induced increases in bronchiolar mast cells and to determine whether virus-induced increases in bronchiolar mast cells were associated with increased airway responsiveness to methacholine and with altered allergic inflammatory responses to antigen stimulation. Mast cell density in intrapulmonary airways was measured in outbred CD (Crl:CDBR) rats by use of morphometric techniques at 7, 15, 30, 60, and 90 days after viral or sham inoculation. Density of bronchiolar mast cells was higher in virus-inoculated rats than in control rats at 30, 60, and 90 days after inoculation (P less than 0.01), but not at 7 or 15 days after inoculation. Total pulmonary mast cell numbers were increased in virus-inoculated rats at 30 days after inoculation. Rats at 42 days after viral inoculation had over a threefold increase in sensitivity to the concentration of nebulized metbacholine that would stimulate a 50% increase in respiratory resistance. Virus-inoculated rats sensitized to ovalbumin had over a 10-fold increase (P less than 0.02) in pulmonary neutrophils that were recovered by bronchoalveolar lavage at 4 hours after ovalbumin aerosol challenge. Virus-inoculated rats at this time also had higher densities of neutrophils in bronchiolar walls than allergen-exposed control rats. The results indicate that Sendai virus induces increases in numbers of bronchiolar mast cells at times from 30 to 90 days after inoculation, and that mast cell increases are associated with airway hyperresponsiveness to methacholine and heightened allergic airway inflammatory reactions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachelet C. M., Bernaudin J. F., Fleury-Feith J. Distribution and histochemical characterization of pulmonary mast cells in the rat and guinea pig. Int Arch Allergy Appl Immunol. 1988;87(3):225–229. doi: 10.1159/000234677. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. New concepts in the pathogenesis of bronchial hyperresponsiveness and asthma. J Allergy Clin Immunol. 1989 Jun;83(6):1013–1026. doi: 10.1016/0091-6749(89)90441-7. [DOI] [PubMed] [Google Scholar]
- Barrett K. E., Metcalfe D. D. Heterogeneity of mast cells in the tissues of the respiratory tract and other organ systems. Am Rev Respir Dis. 1987 May;135(5):1190–1195. doi: 10.1164/arrd.1987.135.5.1190. [DOI] [PubMed] [Google Scholar]
- Bienenstock J., Tomioka M., Stead R., Ernst P., Jordana M., Gauldie J., Dolovich J., Denburg J. Mast cell involvement in various inflammatory processes. Am Rev Respir Dis. 1987 Jun;135(6 Pt 2):S5–S8. doi: 10.1164/arrd.1987.135.6P2.S5. [DOI] [PubMed] [Google Scholar]
- Blythe S., England D., Esser B., Junk P., Lemanske R. F., Jr IgE antibody mediated inflammation of rat lung: histologic and bronchoalveolar lavage assessment. Am Rev Respir Dis. 1986 Dec;134(6):1246–1251. doi: 10.1164/arrd.1986.134.5.1246. [DOI] [PubMed] [Google Scholar]
- Buckner C. K., Songsiridej V., Dick E. C., Busse W. W. In vivo and in vitro studies on the use of the guinea pig as a model for virus-provoked airway hyperreactivity. Am Rev Respir Dis. 1985 Aug;132(2):305–310. doi: 10.1164/arrd.1985.132.2.305. [DOI] [PubMed] [Google Scholar]
- Castleman W. L. Alterations in pulmonary ultrastructure and morphometric parameters induced by parainfluenza (Sendai) virus in rats during postnatal growth. Am J Pathol. 1984 Feb;114(2):322–335. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L. Bronchiolitis obliterans and pneumonia induced in young dogs by experimental adenovirus infection. Am J Pathol. 1985 Jun;119(3):495–504. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Brundage-Anguish L. J., Kreitzer L., Neuenschwander S. B. Pathogenesis of bronchiolitis and pneumonia induced in neonatal and weanling rats by parainfluenza (Sendai) virus. Am J Pathol. 1987 Nov;129(2):277–286. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Northrop P. J., McAllister P. K. Replication of parainfluenza (Sendai) virus in isolated rat pulmonary type II alveolar epithelial cells. Am J Pathol. 1989 May;134(5):1135–1142. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Owens S. B., Brundage-Anguish L. J. Acute and persistent alterations in pulmonary inflammatory cells and airway mast cells induced by Sendai virus infection in neonatal rats. Vet Pathol. 1989 Jan;26(1):18–25. doi: 10.1177/030098588902600104. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Sorkness R. L., Lemanske R. F., Grasee G., Suyemoto M. M. Neonatal viral bronchiolitis and pneumonia induces bronchiolar hypoplasia and alveolar dysplasia in rats. Lab Invest. 1988 Sep;59(3):387–396. [PubMed] [Google Scholar]
- Caughey G. H. Roles of mast cell tryptase and chymase in airway function. Am J Physiol. 1989 Aug;257(2 Pt 1):L39–L46. doi: 10.1152/ajplung.1989.257.2.L39. [DOI] [PubMed] [Google Scholar]
- Coates D. M., Husseini R. H., Collie M. H., Sweet C., Smith H. The role of cellular susceptibility in the declining severity of respiratory influenza of ferrets with age. Br J Exp Pathol. 1984 Feb;65(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Model systems for studying the pathogenesis of infections causing bronchiolitis in man. Pediatr Res. 1977 Mar;11(3 Pt 2):243–246. [PubMed] [Google Scholar]
- Enerbäck L., Norrby K. The mast cells. Curr Top Pathol. 1989;79:169–204. doi: 10.1007/978-3-642-73855-5_8. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Dixon C. M., Dollery C. T., Barnes P. J. Prostaglandin D2 potentiates airway responsiveness to histamine and methacholine. Am Rev Respir Dis. 1986 Feb;133(2):252–254. doi: 10.1164/arrd.1986.133.2.252. [DOI] [PubMed] [Google Scholar]
- Glezen W. P. Reactive airway disorders in children. Role of respiratory virus infections. Clin Chest Med. 1984 Dec;5(4):635–643. [PubMed] [Google Scholar]
- Goto T., Befus D., Low R., Bienenstock J. Mast cell heterogeneity and hyperplasia in bleomycin-induced pulmonary fibrosis of rats. Am Rev Respir Dis. 1984 Nov;130(5):797–802. doi: 10.1164/arrd.1984.130.5.797. [DOI] [PubMed] [Google Scholar]
- Holgate S. T., Kay A. B. Mast cells, mediators and asthma. Clin Allergy. 1985 May;15(3):221–234. [PubMed] [Google Scholar]
- Iwai H., Machii K., Otsuka Y., Ueda K. T cells subsets responsible for clearance of Sendai virus from infected mouse lungs. Microbiol Immunol. 1988;32(3):305–315. doi: 10.1111/j.1348-0421.1988.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Katunuma N., Kido H. Biological functions of serine proteases in mast cells in allergic inflammation. J Cell Biochem. 1988 Dec;38(4):291–301. doi: 10.1002/jcb.240380408. [DOI] [PubMed] [Google Scholar]
- Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Prout S. J., Jaffery G., Bell E. B. Induction of an auto-anti-IgE response in rats. II. Effects on mast cell populations. Eur J Immunol. 1987 Apr;17(4):445–451. doi: 10.1002/eji.1830170402. [DOI] [PubMed] [Google Scholar]
- McConnochie K. M., Roghmann K. J. Bronchiolitis as a possible cause of wheezing in childhood: new evidence. Pediatrics. 1984 Jul;74(1):1–10. [PubMed] [Google Scholar]
- Mok J. Y., Simpson H. Outcome for acute bronchitis, bronchiolitis, and pneumonia in infancy. Arch Dis Child. 1984 Apr;59(4):306–309. doi: 10.1136/adc.59.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Horswood R. L., Camargo E., Chanock R. M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978 Dec;93(3):771–791. [PMC free article] [PubMed] [Google Scholar]
- Reid L. Influence of the pattern of structural growth of lung on susceptibility to specific infectious diseases in infants and children. Pediatr Res. 1977 Mar;11(3 Pt 2):210–215. [PubMed] [Google Scholar]
- Sekizawa K., Caughey G. H., Lazarus S. C., Gold W. M., Nadel J. A. Mast cell tryptase causes airway smooth muscle hyperresponsiveness in dogs. J Clin Invest. 1989 Jan;83(1):175–179. doi: 10.1172/JCI113855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig L. M., Busse W. W., Lemen R. J., Ram J. S. NHLBI workshop summary. Models of infectious airway injury in children. Am Rev Respir Dis. 1988 Apr;137(4):979–984. doi: 10.1164/ajrccm/137.4.979. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hughes M., Collins A. P. Respiratory syncytial virus infection in mice. Infect Immun. 1984 Feb;43(2):649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M., Goto T., Lee T. D., Bienenstock J., Befus A. D. Isolation and characterization of lung mast cells from rats with bleomycin-induced pulmonary fibrosis. Immunology. 1989 Mar;66(3):439–444. [PMC free article] [PubMed] [Google Scholar]
- Wagener J. S., Minnich L., Sobonya R., Taussig L. M., Ray C. G., Fulginiti V. Parainfluenza type II infection in dogs. A model for viral lower respiratory tract infection in humans. Am Rev Respir Dis. 1983 Jun;127(6):771–775. doi: 10.1164/arrd.1983.127.6.771. [DOI] [PubMed] [Google Scholar]
- Weiss S. T., Tager I. B., Muñoz A., Speizer F. E. The relationship of respiratory infections in early childhood to the occurrence of increased levels of bronchial responsiveness and atopy. Am Rev Respir Dis. 1985 Apr;131(4):573–578. doi: 10.1164/arrd.1985.131.4.573. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Wong D. T., Sun M., McCarthy N. Parainfluenza virus bronchiolitis. Epidemiology and pathogenesis. Am J Dis Child. 1986 Jan;140(1):34–40. doi: 10.1001/archpedi.1986.02140150036029. [DOI] [PubMed] [Google Scholar]
- Wingren U., Enerbäck L. Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem J. 1983 Jun;15(6):571–582. doi: 10.1007/BF01954148. [DOI] [PubMed] [Google Scholar]
- Wohl M. E., Chernick V. State of the art: bronchiolitis. Am Rev Respir Dis. 1978 Oct;118(4):759–781. doi: 10.1164/arrd.1978.118.4.759. [DOI] [PubMed] [Google Scholar]