Abstract
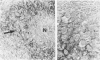
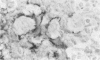
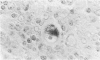
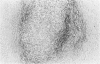
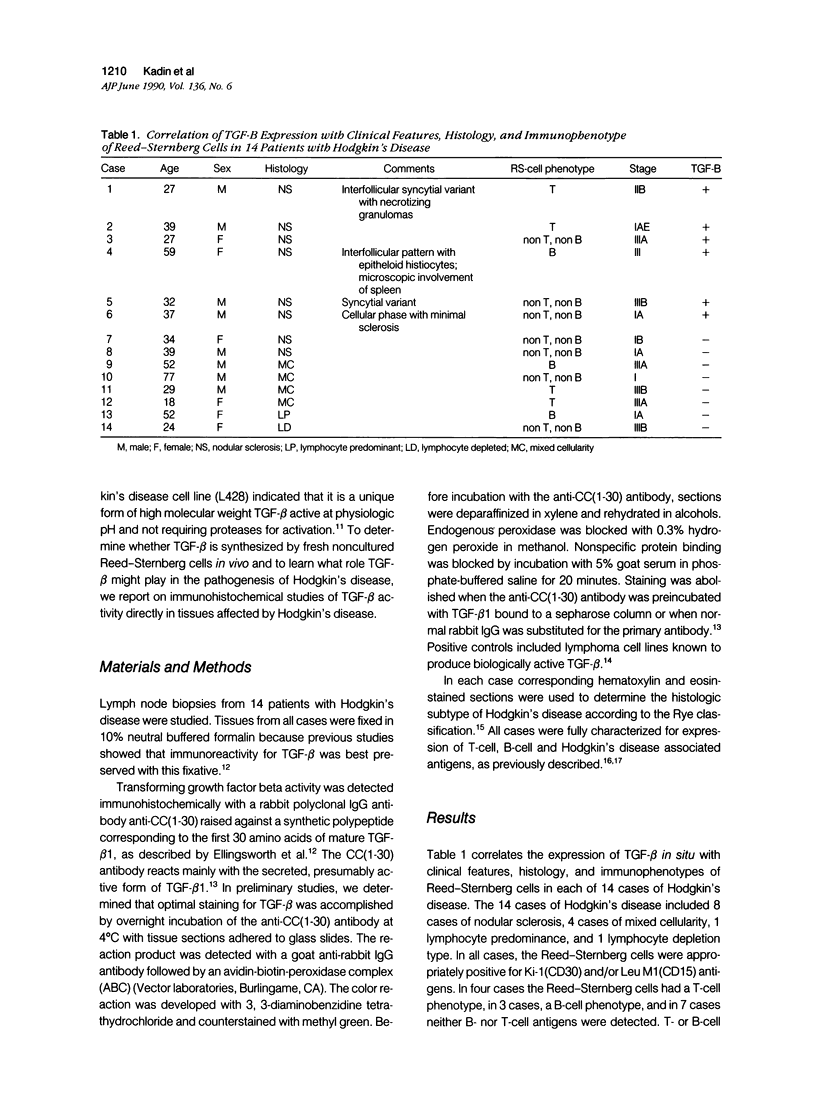
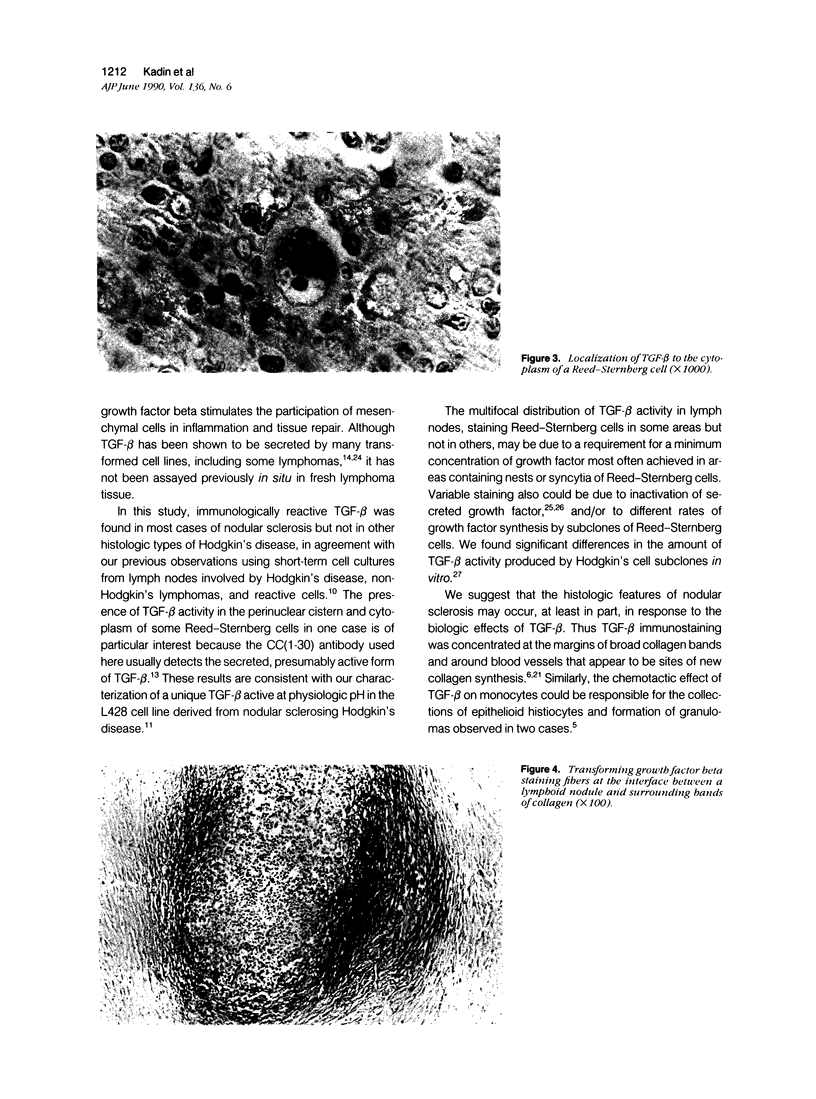
Transforming growth factor beta (TFG-beta) is a multifunctional growth factor that promotes the growth of fibroblasts, collagen synthesis and angiogenesis, and stimulates monocyte migration and activation, but suppresses the growth and differentiation of immune lymphocytes and killer cells. Previously we demonstrated biologic activity for TGF-beta in supernatants of fresh Hodgkin's disease (HD) cell cultures and the cell line L428 derived from nodular sclerosing HD. This study was undertaken to find evidence of TGF-beta activity directly in tissues affected by HD. Formalin-fixed tissue from 14 patients with HD, including 8 nodular sclerosis, 4 mixed cellularity, 1 lymphocyte predominance, and 1 lymphocyte depletion type were studied by immunoperoxidase technique with antibody CC (1-30) raised against a synthetic polypeptide with the same N-terminal amino acid sequence as TGF-beta 1. Transforming growth factor beta activity was demonstrated in six cases of nodular sclerosis but not in other histologic types of HD. Staining for TGF-beta was found in the cytoplasm of Reed-Sternberg (RS) cells in one case and on the surface of RS cells and their lacunar variants in five cases. Transforming growth factor beta activity associated with the extracellular matrix was localized mainly around blood vessels, zones of necrosis, at the margins of bands of collagen sclerosis, and in areas containing syncytia of RS cells. In two cases TGF-beta was associated with collections of epithelioid histiocytes or granulomas. Small lymphocytes, granulocytes, and germinal center cells were unreactive. These results suggest that TGF-beta is a growth factor of biologic importance in HD and may be responsible for many of the histologic features, such as nodular sclerosis and granulomas, that may have prognostic significance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnarsson B. A., Kadin M. E. The immunophenotype of Reed-Sternberg cells. A study of 50 cases of Hodgkin's disease using fixed frozen tissues. Cancer. 1989 Jun 1;63(11):2083–2087. doi: 10.1002/1097-0142(19890601)63:11<2083::aid-cncr2820631102>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Kost L. J., Lyons R. M., Moses H. L., LaRusso N. F. Hepatic processing of transforming growth factor beta in the rat. Uptake, metabolism, and biliary excretion. J Clin Invest. 1987 Sep;80(3):750–757. doi: 10.1172/JCI113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Falini B., Stein H., Pileri S., Canino S., Farabbi R., Martelli M. F., Grignani F., Fagioli M., Minelli O., Ciani C. Expression of lymphoid-associated antigens on Hodgkin's and Reed-Sternberg cells of Hodgkin's disease. An immunocytochemical study on lymph node cytospins using monoclonal antibodies. Histopathology. 1987 Dec;11(12):1229–1242. doi: 10.1111/j.1365-2559.1987.tb01869.x. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Thompson N. L., Cissel D. S., Van Obberghen-Schilling E., Baker C. C., Kass M. E., Ellingsworth L. R., Roberts A. B., Sporn M. B. Transforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989 Feb;108(2):653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Glatstein E., Dorfman R. F. Clinicopathologic studies of 117 untreated patients subjected to laparotomy for the staging of Hodgkin's disease. Cancer. 1971 Jun;27(6):1277–1294. doi: 10.1002/1097-0142(197106)27:6<1277::aid-cncr2820270602>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Kadin M. E., Muramoto L., Said J. Expression of T-cell antigens on Reed-Sternberg cells in a subset of patients with nodular sclerosing and mixed cellularity Hodgkin's disease. Am J Pathol. 1988 Feb;130(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Kasid A., Bell G. I., Director E. P. Effects of transforming growth factor-beta on human lymphokine-activated killer cell precursors. Autocrine inhibition of cellular proliferation and differentiation to immune killer cells. J Immunol. 1988 Jul 15;141(2):690–698. [PubMed] [Google Scholar]
- Kehrl J. H., Roberts A. B., Wakefield L. M., Jakowlew S., Sporn M. B., Fauci A. S. Transforming growth factor beta is an important immunomodulatory protein for human B lymphocytes. J Immunol. 1986 Dec 15;137(12):3855–3860. [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Kuppner M. C., Hamou M. F., Bodmer S., Fontana A., de Tribolet N. The glioblastoma-derived T-cell suppressor factor/transforming growth factor beta 2 inhibits the generation of lymphokine-activated killer (LAK) cells. Int J Cancer. 1988 Oct 15;42(4):562–567. doi: 10.1002/ijc.2910420416. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A., Diehl V. L-428 nodular sclerosing Hodgkin's cell secretes a unique transforming growth factor-beta active at physiologic pH. J Clin Invest. 1988 Dec;82(6):1915–1921. doi: 10.1172/JCI113810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Ansari A. A. Production of transforming growth factor-beta activity by Ki-1 positive lymphoma cells and analysis of its role in the regulation of Ki-1 positive lymphoma growth. Am J Pathol. 1988 Jun;131(3):569–577. [PMC free article] [PubMed] [Google Scholar]
- Newcom S. R., Kadin M. E., Phillips C. L-428 Reed-Sternberg cells and mononuclear Hodgkin's cells arise from a single cloned mononuclear cell. Int J Cell Cloning. 1988 Nov;6(6):417–431. doi: 10.1002/stem.5530060606. [DOI] [PubMed] [Google Scholar]
- Newcom S. R., O'Rourke L. Potentiation of fibroblast growth by nodular sclerosing Hodgkin's disease cell cultures. Blood. 1982 Jul;60(1):228–237. [PubMed] [Google Scholar]
- Newcom S. R. The Hodgkin's cell in nodular sclerosis does not release interleukin-1. J Lab Clin Med. 1985 Feb;105(2):170–177. [PubMed] [Google Scholar]
- O'Connor-McCourt M. D., Wakefield L. M. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987 Oct 15;262(29):14090–14099. [PubMed] [Google Scholar]
- Pinkus G. S., Said J. W. Hodgkin's disease, lymphocyte predominance type, nodular--further evidence for a B cell derivation. L & H variants of Reed-Sternberg cells express L26, a pan B cell marker. Am J Pathol. 1988 Nov;133(2):211–217. [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook A. H., Kehrl J. H., Wakefield L. M., Roberts A. B., Sporn M. B., Burlington D. B., Lane H. C., Fauci A. S. Effects of transforming growth factor beta on the functions of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol. 1986 May 15;136(10):3916–3920. [PubMed] [Google Scholar]
- Sheibani K., Winberg C. D., van de Velde S., Blayney D. W., Rappaport H. Distribution of lymphocytes with interleukin-2 receptors (TAC antigens) in reactive lymphoproliferative processes, Hodgkin's disease, and non-Hodgkin's lymphomas. An immunohistologic study of 300 cases. Am J Pathol. 1987 Apr;127(1):27–37. [PMC free article] [PubMed] [Google Scholar]
- Twomey J. J., Rice L. Impact of Hodgkin's disease upon the immune system. Semin Oncol. 1980 Jun;7(2):114–125. [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]