Abstract
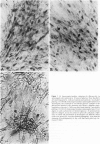
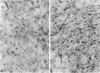
Diabetic nephropathy is a major cause of the increased morbidity and mortality in insulin-dependent diabetes mellitus. The most significant renal lesion of diabetic nephropathy is expansion of the glomerular mesangium. Thickening of the glomerular basement membrance is also apparent. Mesangial expansion is largely due to the accumulation of extracellular matrix (ECM) proteins such as fibronectin, laminin, and type IV collagen. To determine whether high glucose is responsible for the observed increase in mesangial cell ECM protein accumulation, mesangial cells were grown in tissue culture medium containing 10 mmol/l (millimolar) glucose (normal) or 30 mmol/l glucose (high). The degree of ECM protein accumulation was determined by immunocytochemistry and a solid-phase enzyme-linked immunosorbent assay (ELISA) developed in the laboratory. Mesangial cells cultured for 1 week contained fibronectin as the most abundant ECM protein, followed by laminin and type IV collagen. Type IV collagen was seen only after the cells had piled up into 'hillocks' (approximately 4 weeks of continuous growth without passaging). After 4 weeks in 30 mmol/l glucose, mesangial cells contained increased amounts of all three matrix proteins. Fibronectin and laminin were increased by approximately 60%, while type IV collagen was increased 50%. Cells subcultured in medium containing 30 mmol/l glucose for 8 months displayed a twofold increase in fibronectin and laminin. Thus, high glucose per se can cause changes in mesangial cell ECM. This cell culture model should be useful in elucidating the mechanisms involved.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrass C. K., Peterson C. V., Raugi G. J. Phenotypic expression of collagen types in mesangial matrix of diabetic and nondiabetic rats. Diabetes. 1988 Dec;37(12):1695–1702. doi: 10.2337/diab.37.12.1695. [DOI] [PubMed] [Google Scholar]
- Allen-Hoffmann B. L., Mosher D. F. Matrix assembly sites for exogenous fibronectin are decreased on human fibroblasts after treatment with agents which increase intracellular cAMP. J Biol Chem. 1987 Oct 15;262(29):14361–14365. [PubMed] [Google Scholar]
- Ausiello D. A., Kreisberg J. I., Roy C., Karnovsky M. J. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980 Mar;65(3):754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M., Vlassara H., Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984 Oct;101(4):527–537. doi: 10.7326/0003-4819-101-4-527. [DOI] [PubMed] [Google Scholar]
- Bruneval P., Foidart J. M., Nochy D., Camilleri J. P., Bariety J. Glomerular matrix proteins in nodular glomerulosclerosis in association with light chain deposition disease and diabetes mellitus. Hum Pathol. 1985 May;16(5):477–484. doi: 10.1016/s0046-8177(85)80086-1. [DOI] [PubMed] [Google Scholar]
- Cagliero E., Maiello M., Boeri D., Roy S., Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988 Aug;82(2):735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. P., Khalifa A. Effect of diabetes and insulin on rat renal glomerular protocollagen hydroxylase activities. Biochim Biophys Acta. 1977 Jan 24;496(1):88–94. doi: 10.1016/0304-4165(77)90117-9. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Khalifa A. Renal glomerular collagen synthesis in streptozotocin diabetes. Reversal of increased basement membrane synthesis with insulin therapy. Biochim Biophys Acta. 1977 Dec 22;500(2):395–404. doi: 10.1016/0304-4165(77)90030-7. [DOI] [PubMed] [Google Scholar]
- Cohen M. P., Surma M. L., Wu V. Y. In vivo biosynthesis and turnover of glomerular basement membrane in diabetic rats. Am J Physiol. 1982 Apr;242(4):F385–F389. doi: 10.1152/ajprenal.1982.242.4.F385. [DOI] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- GELLMAN D. D., PIRANI C. L., SOOTHILL J. F., MUEHRCKE R. C., MADUROS W., KARK R. M. Structure and function in diabetic nephropathy; the importance of diffuse glomerulosclerosis. Diabetes. 1959 Jul-Aug;8(4):251–256. doi: 10.2337/diab.8.4.251. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Radnik R. A., Garoni J. A., Kreisberg J. I. Urokinase-dependent adhesion loss and shape change after cyclic adenosine monophosphate elevation in cultured rat mesangial cells. J Clin Invest. 1988 Dec;82(6):1992–2000. doi: 10.1172/JCI113819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka K., McCoy J., Kimmelsteil P. The glomerular mesangium. A quantitative analysis. Lab Invest. 1968 Dec;19(6):573–579. [PubMed] [Google Scholar]
- Ishimura E., Sterzel R. B., Budde K., Kashgarian M. Formation of extracellular matrix by cultured rat mesangial cells. Am J Pathol. 1989 Apr;134(4):843–855. [PMC free article] [PubMed] [Google Scholar]
- Kawano K., Arakawa M., McCoy J., Porch J., Kimmelstiel P. Quantitative study of glomeruli. Focal glomerulonephritis and diabetic glomerulosclerosis. Lab Invest. 1969 Sep;21(3):269–275. [PubMed] [Google Scholar]
- Kilo C. Value of glucose control in preventing complications of diabetes. Am J Med. 1985 Aug 23;79(2B):33–37. doi: 10.1016/0002-9343(85)90583-2. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M. A., Patel P. Y. Cyclic AMP-associated shape change in mesangial cells and its reversal by prostaglandin E2. Kidney Int. 1984 Jun;25(6):874–879. doi: 10.1038/ki.1984.104. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Venkatachalam M. A. Vasoactive agents affect mesangial cell adhesion. Am J Physiol. 1986 Oct;251(4 Pt 1):C505–C511. doi: 10.1152/ajpcell.1986.251.4.C505. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B. Cell culture approaches to the analysis of glomerular inflammation. Kidney Int. 1986 Aug;30(2):246–254. doi: 10.1038/ki.1986.176. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Sterzel R. B., Kashgarian M., Ryan J. L. Neutral proteinase activity produced in vitro by cells of the glomerular mesangium. Kidney Int. 1983 Feb;23(2):342–349. doi: 10.1038/ki.1983.25. [DOI] [PubMed] [Google Scholar]
- Lubec G., Pollak A. Reduced susceptibility of nonenzymatically glucosylated glomerular basement membrane to proteases: is thickening of diabetic glomerular basement membranes due to reduced proteolytic degradation? Ren Physiol. 1980;3(1-6):4–8. doi: 10.1159/000172733. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer S. M., Steffes M. W., Ellis E. N., Sutherland D. E., Brown D. M., Goetz F. C. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984 Oct;74(4):1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo P. J., Mosher D. F. Binding of plasma fibronectin to cell layers of human skin fibroblasts. J Cell Biol. 1983 Aug;97(2):466–472. doi: 10.1083/jcb.97.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. Renal function changes in diabetes. Diabetes. 1976;25(2 Suppl):872–879. [PubMed] [Google Scholar]
- Phan-Thanh L., Robert L., Derouette J. C., Labat-Robert J. Increased biosynthesis and processing of fibronectin in fibroblasts from diabetic mice. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1911–1915. doi: 10.1073/pnas.84.7.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R., Kurkinen M., Prockop D. J., Boot-Handford R. P. Increased steady-state levels of laminin B1 mRNA in kidneys of long-term streptozotocin-diabetic rats. No effect of an aldose reductase inhibitor. J Biol Chem. 1988 Jul 25;263(21):10072–10076. [PubMed] [Google Scholar]
- Scheinman J. I., Fish A. J., Matas A. J., Michael A. F. The immunohistopathology of glomerular antigens. II. The glomerular basement membrane, actomyosin, and fibroblast surface antigens in normal, diseased, and transplanted human kidneys. Am J Pathol. 1978 Jan;90(1):71–88. [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steffes M. W., Brown D. M., Basgen J. M., Mauer S. M. Amelioration of mesangial volume and surface alterations following islet transplantation in diabetic rats. Diabetes. 1980 Jul;29(7):509–515. doi: 10.2337/diab.29.7.509. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Lovett D. H., Foellmer H. G., Perfetto M., Biemesderfer D., Kashgarian M. Mesangial cell hillocks. Nodular foci of exaggerated growth of cells and matrix in prolonged culture. Am J Pathol. 1986 Oct;125(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- Viberti G. C., Pickup J. C., Jarrett R. J., Keen H. Effect of control of blood glucose on urinary excretion of albumin and beta2 microglobulin in insulin-dependent diabetes. N Engl J Med. 1979 Mar 22;300(12):638–641. doi: 10.1056/NEJM197903223001202. [DOI] [PubMed] [Google Scholar]