Abstract
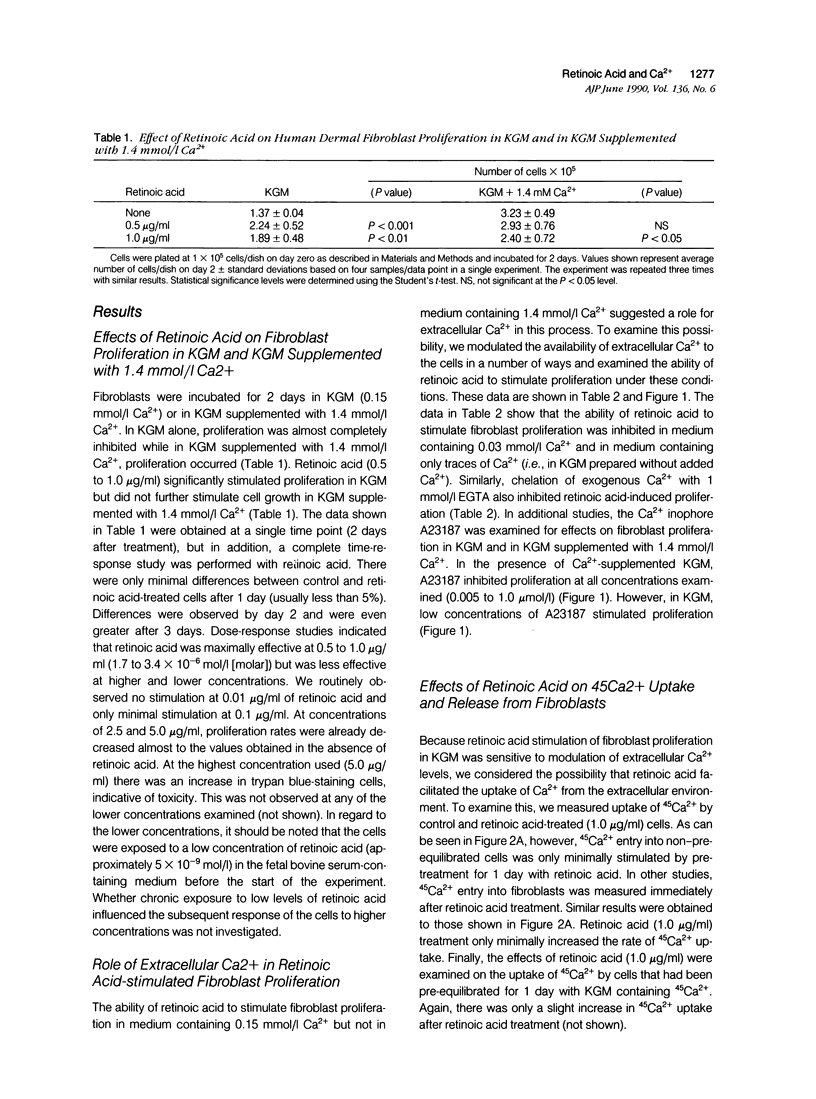
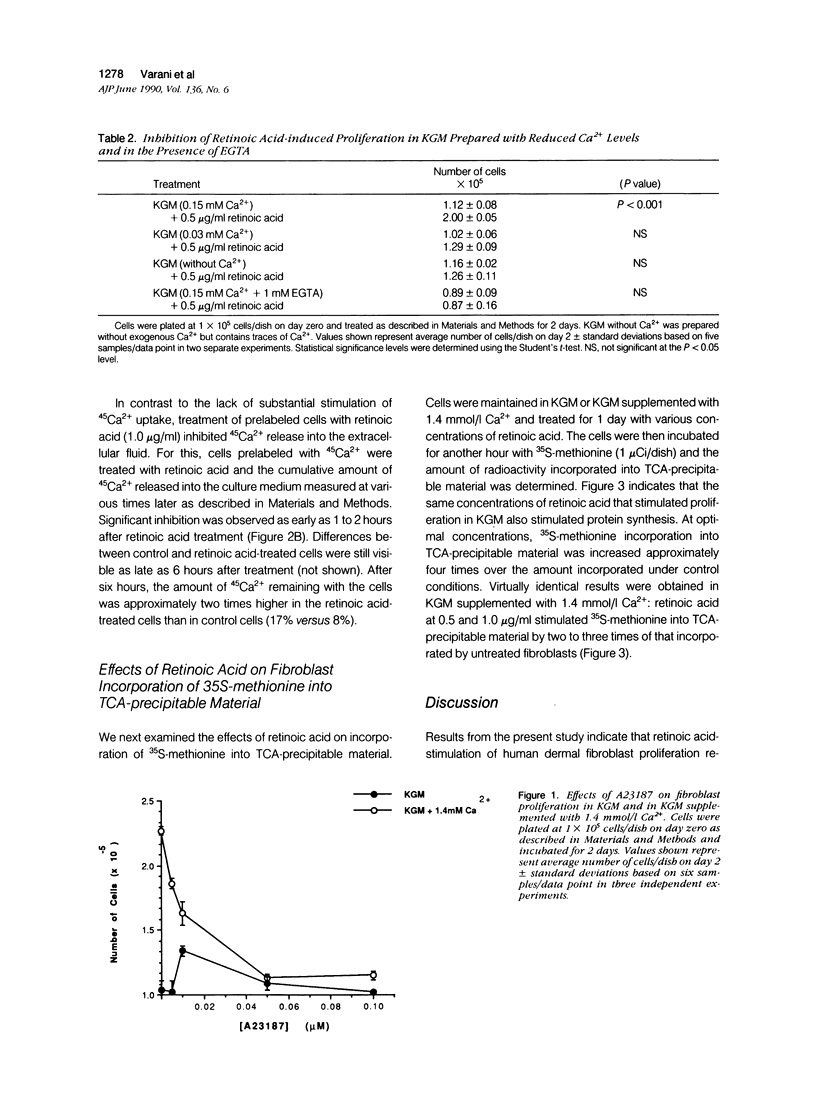
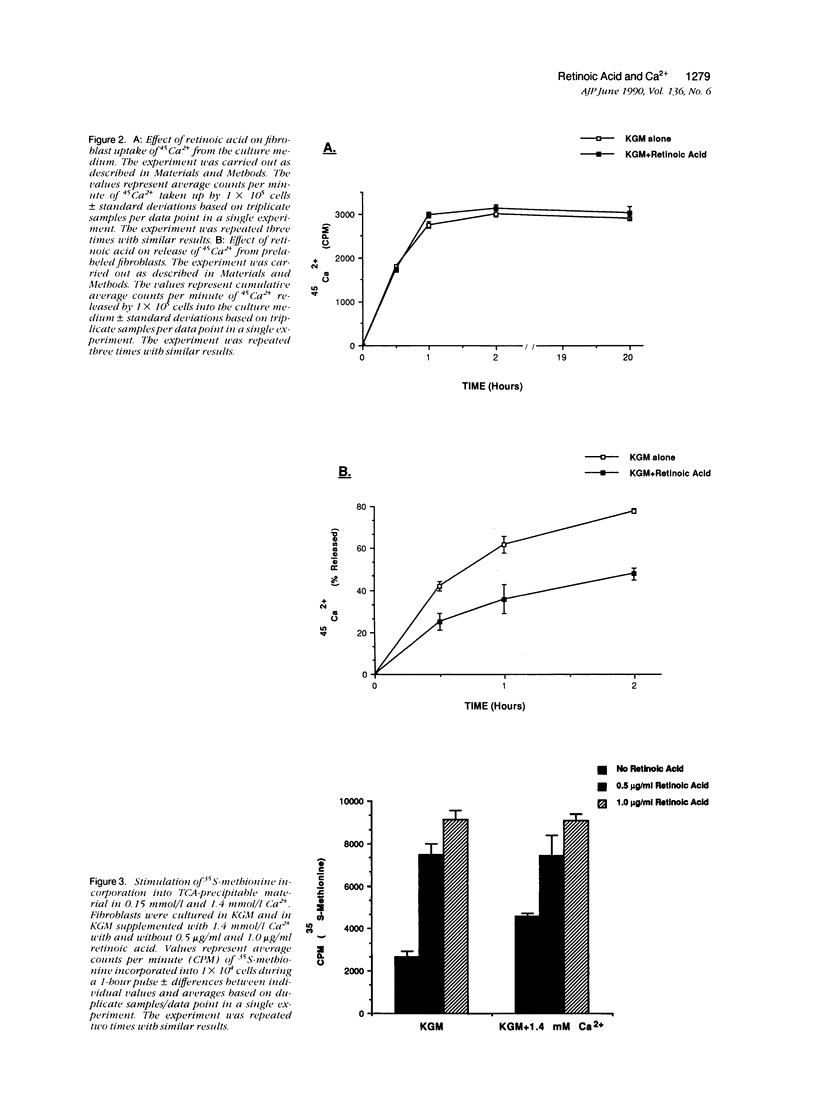
Human dermal fibroblasts failed to proliferate when cultured in medium containing 0.15 mmol/l (millimolar) Ca2+ (keratinocyte growth medium [KGM]) but did when the external Ca2+ concentration was raised to 1.4 mmol/l. All-trans retinoic acid (retinoic acid) stimulated proliferation in KGM but did not further stimulate growth in Ca2(+)-supplemented KGM. The ability of retinoic acid to stimulate proliferation was inhibited in KGM prepared without Ca2+ or prepared with 0.03 mmol/l Ca2+ and in KGM treated with 1 mmol/l ethylene-glycol-bis-(beta-aminoethyl ether)N,N'-tetra acetic acid. Using 45Ca2+ to measure Ca2+ influx and efflux, it was found that retinoic acid minimally increased Ca2+ uptake into fibroblasts. In contrast, retinoic acid treatment of fibroblasts that had been pre-equilibrated for 1 day with 45Ca2+ inhibited release of intracellular Ca2+ into the extracellular fluid. Retinoic acid also stimulated 35S-methionine incorporation into trichloroacetic acid-precipitable material but in contrast to its effect on proliferation, stimulation of 35S-methionine incorporation occurred in both high-Ca2+ and low-Ca2+ medium. These data indicate that retinoic acid stimulation of proliferation, but not protein synthesis, is dependent on the concentration of Ca2+ in the extracellular environment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betsholtz C., Westermark B. Growth factor-induced proliferation of human fibroblasts in serum-free culture depends on cell density and extracellular calcium concentration. J Cell Physiol. 1984 Feb;118(2):203–210. doi: 10.1002/jcp.1041180213. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Tremblay R. The control of human WI-38 cell proliferation by extracellular calcium and its elimination by SV-40 virus-induced proliferative transformation. J Cell Physiol. 1977 Aug;92(2):241–247. doi: 10.1002/jcp.1040920212. [DOI] [PubMed] [Google Scholar]
- Chytil F., Sherman D. R. How do retinoids work? Dermatologica. 1987;175 (Suppl 1):8–12. doi: 10.1159/000248847. [DOI] [PubMed] [Google Scholar]
- Demetriou A. A., Levenson S. M., Rettura G., Seifter E. Vitamin A and retinoic acid: induced fibroblast differentiation in vitro. Surgery. 1985 Nov;98(5):931–934. [PubMed] [Google Scholar]
- Elias P. M., Fritsch P. O., Lampe M., Williams M. L., Brown B. E., Nemanic M., Grayson S. Retinoid effects on epidermal structure, differentiation, and permeability. Lab Invest. 1981 Jun;44(6):531–540. [PubMed] [Google Scholar]
- Fukuo K., Morimoto S., Kaji K., Koh E., Hironaka T., Morita R., Kim S., Onishi T. Association of increased intracellular free Ca2+ by platelet-derived growth factor with mitogenesis but not with proteoglycan synthesis in chondrocytes--effect of suramin. Cell Calcium. 1989 Jan;10(1):29–35. doi: 10.1016/0143-4160(89)90041-9. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harper R. A., Burgoon T. Differential effects of retinoic acid on the growth of normal fibroblast-like cells in vitro from human, swine and rabbit skin. Cell Biol Int Rep. 1982 Feb;6(2):163–170. doi: 10.1016/0309-1651(82)90093-5. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Nakahata N., Lovenberg T. W., DiGuiseppi J., Herman B., Earp H. S., Harden T. K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J Biol Chem. 1987 Mar 5;262(7):2951–2956. [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Kligman A. M., Grove G. L., Hirose R., Leyden J. J. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Kligman L. H., Duo C. H., Kligman A. M. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12(2):139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kligman L. H. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986 Oct;15(4 Pt 2):779-85, 884-7. doi: 10.1016/s0190-9622(86)70234-x. [DOI] [PubMed] [Google Scholar]
- Lacroix A., Anderson G. D., Lippman M. E. Retinoids and cultured human fibroblasts. Effects on cell growth and presence of cellular retinoic acid-binding protein. Exp Cell Res. 1980 Dec;130(2):339–344. doi: 10.1016/0014-4827(80)90010-5. [DOI] [PubMed] [Google Scholar]
- Lanciotti M., Longone P., Cornaglia-Ferraris P., Ponzoni M. Retinoic acid inhibits phosphatidylinositol turnover only in RA-sensitive while not in RA-resistant human neuroblastoma cells. Biochem Biophys Res Commun. 1989 May 30;161(1):284–289. doi: 10.1016/0006-291x(89)91593-3. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Aerts R. J., Tertoolen L. G., de Laat S. W. The epidermal growth factor-induced calcium signal in A431 cells. J Biol Chem. 1986 Jan 5;261(1):279–284. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Orfanos C. E., Runne U. Tissue changes in psoriatic plaques after oral administration of retinoid. Dermatologica. 1978;157 (Suppl 1):19–25. doi: 10.1159/000250880. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Praeger F. C., Reinlib L., Donowitz M., Gilchrest B. A. [Ca2+]i independent mitogenesis in cultured human fibroblasts revealed by single cell microfluorimetry. Biochem Biophys Res Commun. 1989 Mar 15;159(2):862–870. doi: 10.1016/0006-291x(89)90074-0. [DOI] [PubMed] [Google Scholar]
- Priestley G. C. Proliferation and glycosaminoglycans secretion in fibroblasts from psoriatic skin: differential responses to retinoids. Br J Dermatol. 1987 Nov;117(5):575–583. doi: 10.1111/j.1365-2133.1987.tb07489.x. [DOI] [PubMed] [Google Scholar]
- Spearman T. N., Fontana J. A., Butcher F. R., Durham J. P. Effect of retinoic acid on the phosphorylation of endogenous proteins in HL-60 cells. J Cell Physiol. 1989 Feb;138(2):349–357. doi: 10.1002/jcp.1041380218. [DOI] [PubMed] [Google Scholar]
- Stanulis-Praeger B. M., Gilchrest B. A. Effect of donor age and prior sun exposure on growth inhibition of cultured human dermal fibroblasts by all trans-retinoic acid. J Cell Physiol. 1989 Apr;139(1):116–124. doi: 10.1002/jcp.1041390117. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Dixit V. M., Mitra R. S., Voorhees J. J. All-trans retinoic acid stimulates growth of adult human keratinocytes cultured in growth factor-deficient medium, inhibits production of thrombospondin and fibronectin, and reduces adhesion. J Invest Dermatol. 1989 Oct;93(4):449–454. doi: 10.1111/1523-1747.ep12284020. [DOI] [PubMed] [Google Scholar]
- Weiss J. S., Ellis C. N., Headington J. T., Tincoff T., Hamilton T. A., Voorhees J. J. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22;259(4):527–532. [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Williams M. L., Elias P. M. Nature of skin fragility in patients receiving retinoids for systemic effect. Arch Dermatol. 1981 Oct;117(10):611–619. [PubMed] [Google Scholar]


