Abstract
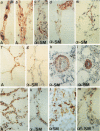
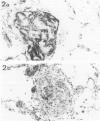
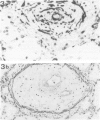
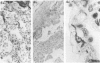
Lungs of 37 patients with pulmonary hypertension (PHT), 5 normal human lungs, and 30 normal rat lungs, were studied using immunohistochemical stainings for actin, alpha-smooth muscle (alpha-SM) actin and desmin. The type of PHT was determined on clinicopathologic grounds (in 17 cases by catheterism); 20 patients had precapillary and 17 postcapillary PHT. In normal lungs, myofibroblasts, ie, contractile interstitial cells (CIC), distributed in alveolar septa, were not stained by alpha-SM actin antibodies. Only around the venules, were cells labeled by this antibody present. Furthermore, there were bundles of alpha-SM actin-positive cells around the openings of air sacculi into the alveolar ducts. In precapillary PHT, the distribution and immunostaining properties of interstitial cells remained unchanged; alpha-SM actin-positive cells were observed in thickened arterial intima and in plexiform lesions. In postcapillary PHT secondary to heart failure, to mitral stenosis, or in veno-occlusive disease, many interstitial cells in the alveolar septa were decorated by alpha-SM actin antibodies but not with desmin. The authors propose that, in postcapillary PHT, mechanical stretch due to capillary congestion may be responsible for the generation of cells that express an actin isoform associated with smooth muscle.
Full text
PDF

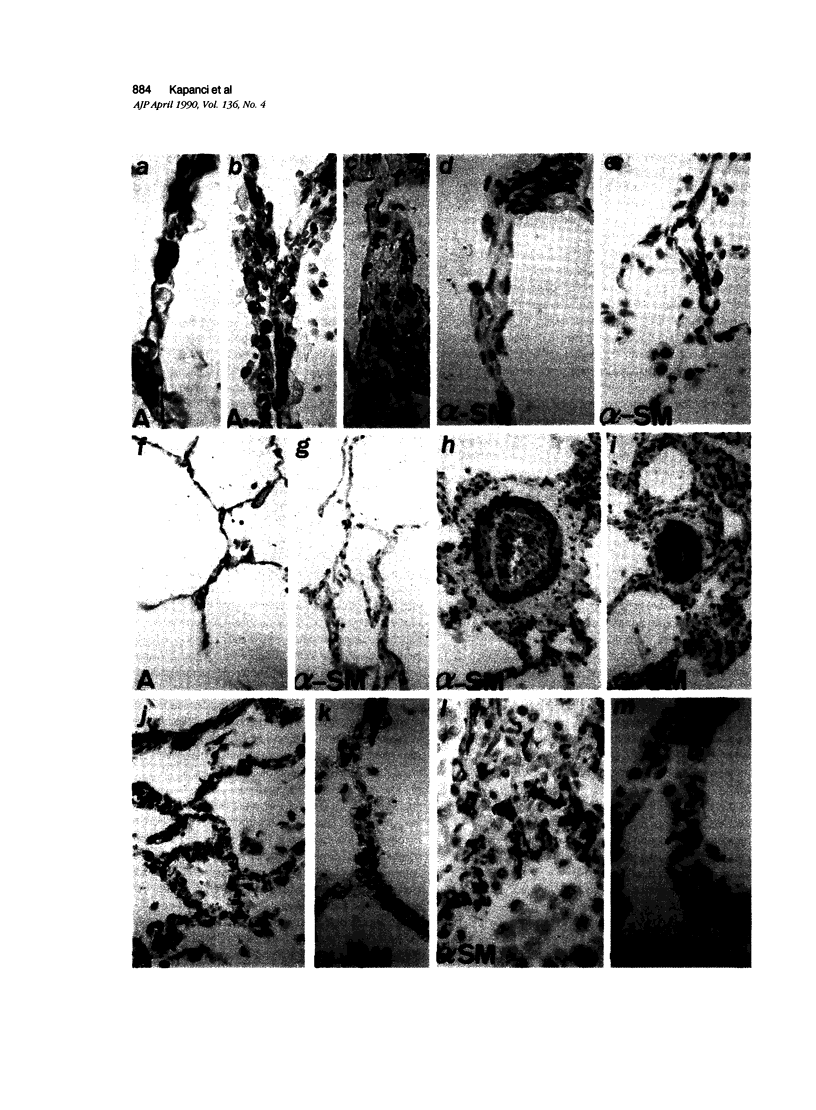

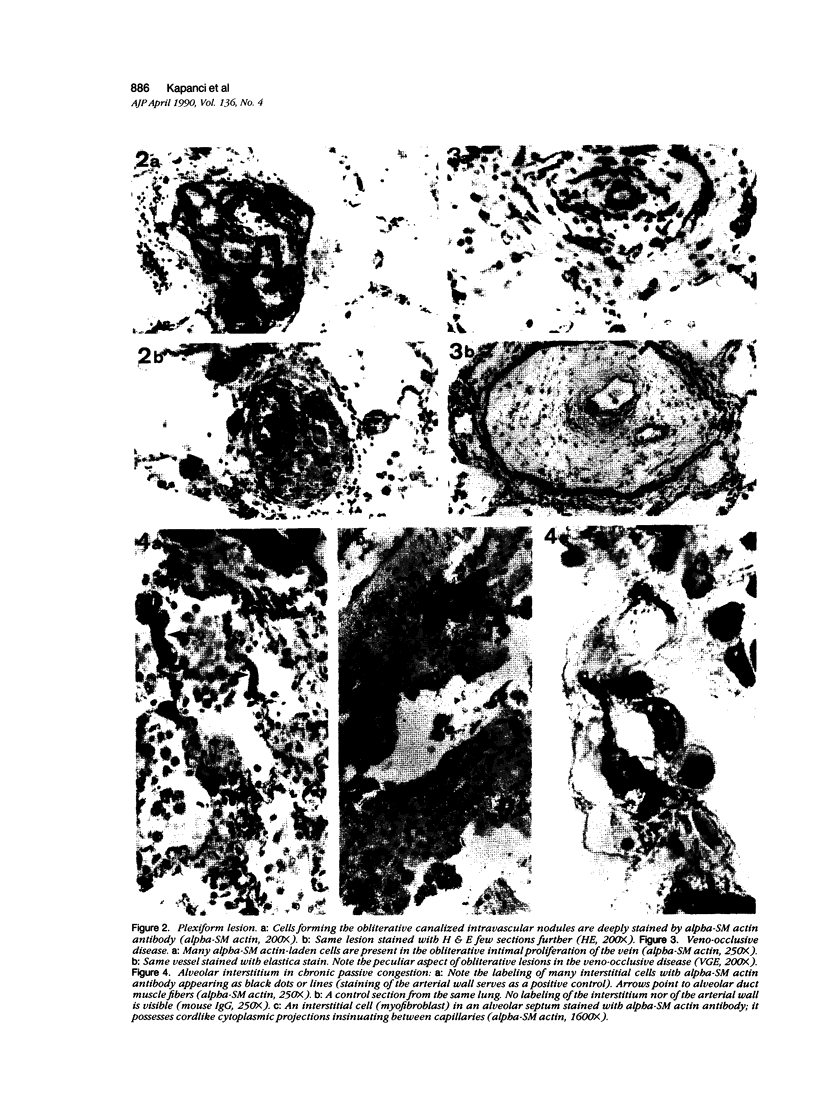



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Callahan L. M., Evans J. N. Cellular alterations in the alveolar wall in bleomycin-induced pulmonary fibrosis in rats. An ultrastructural morphometric study. Am Rev Respir Dis. 1986 Jun;133(6):1043–1048. doi: 10.1164/arrd.1986.133.6.1043. [DOI] [PubMed] [Google Scholar]
- Adler K. B., Craighead J. E., Vallyathan N. V., Evans J. N. Actin-containing cells in human pulmonary fibrosis. Am J Pathol. 1981 Mar;102(3):427–437. [PMC free article] [PubMed] [Google Scholar]
- Adler K. B., Craighead J. E., Vallyathan N. V., Evans J. N. Actin-containing cells in human pulmonary fibrosis. Am J Pathol. 1981 Mar;102(3):427–437. [PMC free article] [PubMed] [Google Scholar]
- Adler K. B., Low R. B., Leslie K. O., Mitchell J., Evans J. N. Contractile cells in normal and fibrotic lung. Lab Invest. 1989 Apr;60(4):473–485. [PubMed] [Google Scholar]
- Allen K. M., Haworth S. G. Cytoskeletal features of immature pulmonary vascular smooth muscle cells: the influence of pulmonary hypertension on normal development. J Pathol. 1989 Aug;158(4):311–317. doi: 10.1002/path.1711580408. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos A., Kapanci Y. Lung fixation by perfusion--a simple method to control the pressure in the perfusion circuit. J Microsc. 1974 Mar;100(2):227–229. doi: 10.1111/j.1365-2818.1974.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Benzonana G., Skalli O., Gabbiani G. Correlation between the distribution of smooth muscle or non muscle myosins and alpha-smooth muscle actin in normal and pathological soft tissues. Cell Motil Cytoskeleton. 1988;11(4):260–274. doi: 10.1002/cm.970110405. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Adelberg S., Crystal R. G. Mechanisms of pulmonary fibrosis. Spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983 Nov;72(5):1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsson J., Edwards W. D. Primary pulmonary hypertension: a histopathologic study of 80 cases. Mayo Clin Proc. 1985 Jan;60(1):16–25. doi: 10.1016/s0025-6196(12)65277-x. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Doane K., McReynolds R. A., Wilson F. J. Immunofluorescence localization of contractile proteins in the rat lung following bleomycin injury. Histochem J. 1983 Jan;15(1):82–88. doi: 10.1007/BF01006074. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Goldstein R. H., Fine A. Fibrotic reactions in the lung: the activation of the lung fibroblast. Exp Lung Res. 1986;11(4):245–261. doi: 10.3109/01902148609062828. [DOI] [PubMed] [Google Scholar]
- Heath D., Smith P., Gosney J., Mulcahy D., Fox K., Yacoub M., Harris P. The pathology of the early and late stages of primary pulmonary hypertension. Br Heart J. 1987 Sep;58(3):204–213. doi: 10.1136/hrt.58.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D., Smith P., Gosney J. Ultrastructure of early plexogenic pulmonary arteriopathy. Histopathology. 1988 Jan;12(1):41–52. doi: 10.1111/j.1365-2559.1988.tb01915.x. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kapanci Y., Assimacopoulos A., Irle C., Zwahlen A., Gabbiani G. "Contractile interstitial cells" in pulmonary alveolar septa: a possible regulator of ventilation-perfusion ratio? Ultrastructural, immunofluorescence, and in vitro studies. J Cell Biol. 1974 Feb;60(2):375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapanci Y., Costabella P. M., Cerutti P., Assimacopoulos A. Distribution and function of cytoskeletal proteins in lung cells with particular reference to 'contractile interstitial cells'. Methods Achiev Exp Pathol. 1979;9:147–168. [PubMed] [Google Scholar]
- Kawanami O., Basset F., Barrios R., Lacronique J. G., Ferrans V. J., Crystal R. G. Hypersensitivity pneumonitis in man. Light- and electron-microscopic studies of 18 lung biopsies. Am J Pathol. 1983 Mar;110(3):275–289. [PMC free article] [PubMed] [Google Scholar]
- Kolega J. Effects of mechanical tension on protrusive activity and microfilament and intermediate filament organization in an epidermal epithelium moving in culture. J Cell Biol. 1986 Apr;102(4):1400–1411. doi: 10.1083/jcb.102.4.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIEBOW A. A., LORING W. E., FELTON W. L., 3rd The musculature of the lungs in chronic pulmonary disease. Am J Pathol. 1953 Sep-Oct;29(5):885–911. [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Immunofluorescence studies on the structure of actin filaments in tissue culture cells. J Histochem Cytochem. 1975 Jul;23(7):507–528. doi: 10.1177/23.7.1095651. [DOI] [PubMed] [Google Scholar]
- Low R. B., Woodcock-Mitchell J., Evans J. N., Adler K. B. Actin content of normal and of bleomycin-fibrotic rat lung. Am Rev Respir Dis. 1984 Feb;129(2):311–316. [PubMed] [Google Scholar]
- Martin J. C., Normand C., Cornu L., Le Bouffant L. E. Le myofibroblaste de la cloison interalvéolaire. Données ultrastructurales. Acta Tuberc Pneumol Belg. 1977 Oct-Dec;68(4):399–410. [PubMed] [Google Scholar]
- Meyrick B., Reid L. The alveolar wall. Br J Dis Chest. 1970 Jul;64(3):121–140. doi: 10.1016/s0007-0971(70)80001-8. [DOI] [PubMed] [Google Scholar]
- Mitchell J., Woodcock-Mitchell J., Reynolds S., Low R., Leslie K., Adler K., Gabbiani G., Skalli O. Alpha-smooth muscle actin in parenchymal cells of bleomycin-injured rat lung. Lab Invest. 1989 May;60(5):643–650. [PubMed] [Google Scholar]
- Pietra G. G., Edwards W. D., Kay J. M., Rich S., Kernis J., Schloo B., Ayres S. M., Bergofsky E. H., Brundage B. H., Detre K. M. Histopathology of primary pulmonary hypertension. A qualitative and quantitative study of pulmonary blood vessels from 58 patients in the National Heart, Lung, and Blood Institute, Primary Pulmonary Hypertension Registry. Circulation. 1989 Nov;80(5):1198–1206. doi: 10.1161/01.cir.80.5.1198. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Structure and function in pulmonary hypertension. New perceptions. Chest. 1986 Feb;89(2):279–288. doi: 10.1378/chest.89.2.279. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Sappino A. P., Skalli O., Jackson B., Schürch W., Gabbiani G. Smooth-muscle differentiation in stromal cells of malignant and non-malignant breast tissues. Int J Cancer. 1988 May 15;41(5):707–712. doi: 10.1002/ijc.2910410512. [DOI] [PubMed] [Google Scholar]
- Schürch W., Seemayer T. A., Lagacé R., Gabbiani G. The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol. 1984;403(4):323–336. doi: 10.1007/BF00737283. [DOI] [PubMed] [Google Scholar]
- Schürch W., Skalli O., Seemayer T. A., Gabbiani G. Intermediate filament proteins and actin isoforms as markers for soft tissue tumor differentiation and origin. I. Smooth muscle tumors. Am J Pathol. 1987 Jul;128(1):91–103. [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Pelte M. F., Peclet M. C., Gabbiani G., Gugliotta P., Bussolati G., Ravazzola M., Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989 Mar;37(3):315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Schürch W., Seemayer T., Lagacé R., Montandon D., Pittet B., Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- Skalli O., Vandekerckhove J., Gabbiani G. Actin-isoform pattern as a marker of normal or pathological smooth-muscle and fibroblastic tissues. Differentiation. 1987;33(3):232–238. doi: 10.1111/j.1432-0436.1987.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Toccanier-Pelte M. F., Skalli O., Kapanci Y., Gabbiani G. Characterization of stromal cells with myoid features in lymph nodes and spleen in normal and pathologic conditions. Am J Pathol. 1987 Oct;129(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Tsilibary E. C., Williams M. C. Actin in peripheral rat lung: S1 labeling and structural changes induced by cytochalasin. J Histochem Cytochem. 1983 Nov;31(11):1289–1297. doi: 10.1177/31.11.6684669. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. On pericytes, particularly their existence on lung capillaries. Microvasc Res. 1974 Sep;8(2):218–235. doi: 10.1016/0026-2862(74)90096-x. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Adler K. B., Low R. B. Immunohistochemical identification of cell types in normal and in bleomycin-induced fibrotic rat lung. Cellular origins of interstitial cells. Am Rev Respir Dis. 1984 Nov;130(5):910–916. doi: 10.1164/arrd.1984.130.5.910. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Mitchell J. J., Low R. B., Kieny M., Sengel P., Rubbia L., Skalli O., Jackson B., Gabbiani G. Alpha-smooth muscle actin is transiently expressed in embryonic rat cardiac and skeletal muscles. Differentiation. 1988 Dec;39(3):161–166. doi: 10.1111/j.1432-0436.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]