Abstract
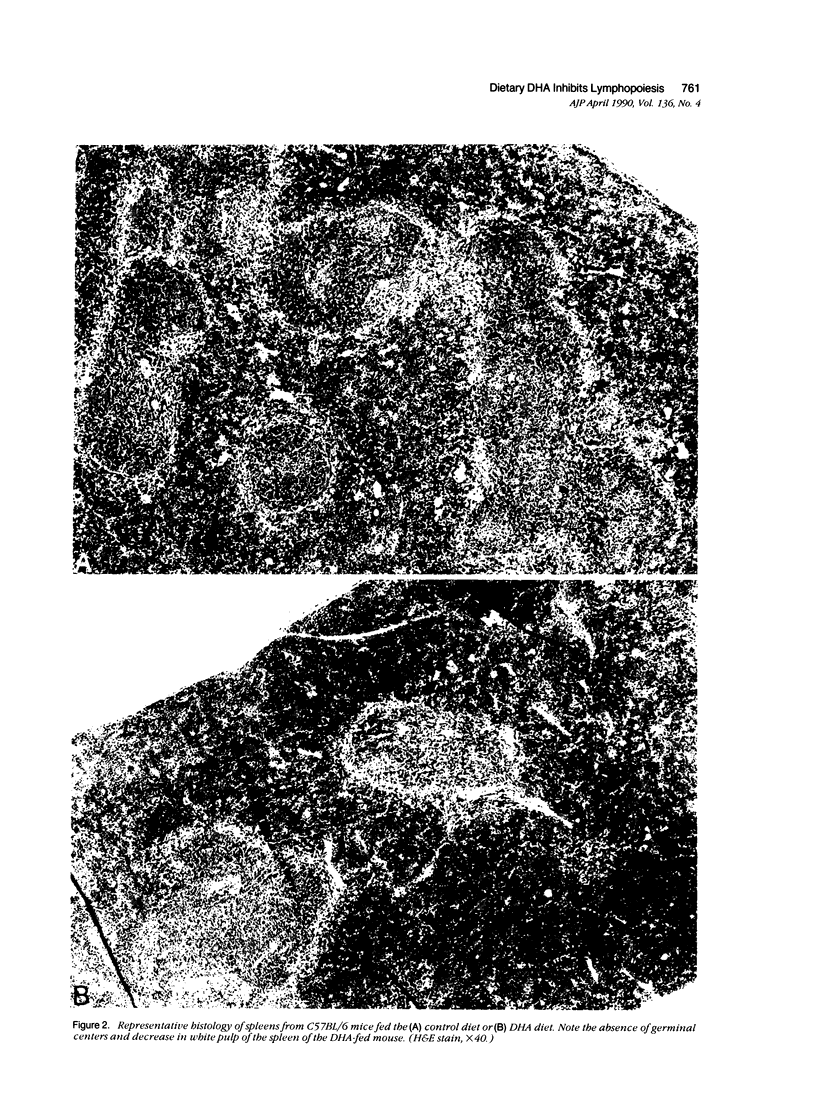
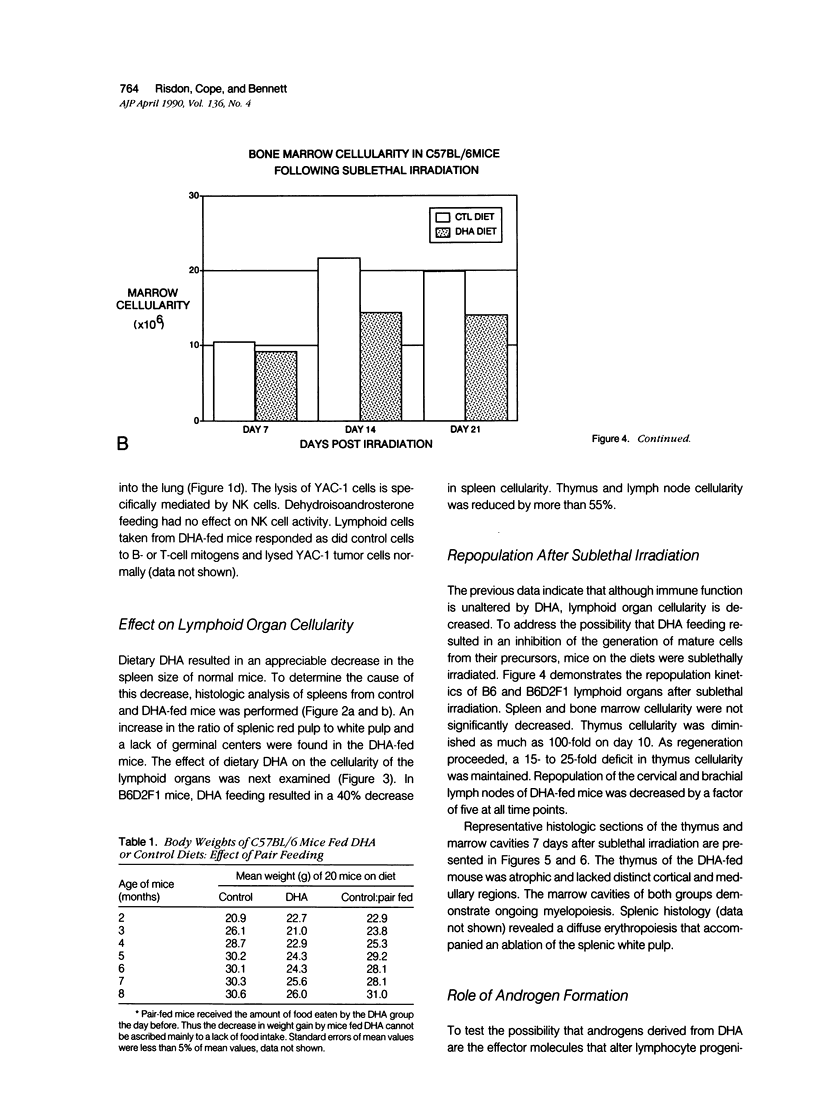
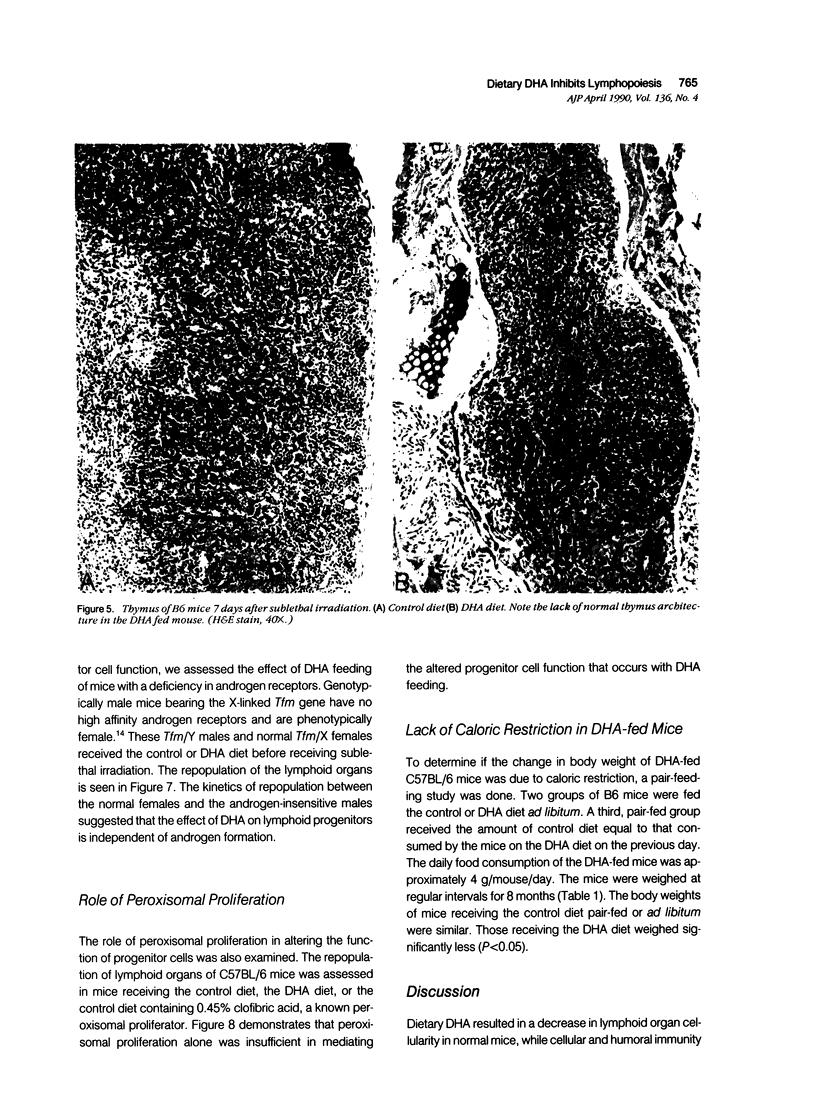
The ingestion of dehydroisoandrosterone (DHA), a naturally occurring steroid, inhibits the development of autoimmunity, neoplasias, and other disorders of rodents. Potential mechanisms of action include (1) the induction of peroxisomal proliferation and (2) the conversion of DHA to androgens. We evaluated the immune system of mice fed DHA. Dietary DHA had no significant effect on antibody responses, cutaneous sensitivity reactions, natural killer activity, or graft-versus-host reactions. However, a decrease in lymphoid organ cellularity and an absence of splenic germinal centers were observed. We assessed progenitor cell activity in irradiated mice by evaluating the repopulation of marrow and lymphoid organs. Dehydroisoandrosterone feeding resulted in an inhibition of lymphopoiesis but not myelopoiesis. Clofibrate, another peroxisomal proliferator, failed to inhibit lymphocyte repopulation after irradiation. Androgen-non-responsive Tfm/Y mice were as susceptible as control mice to the inhibitory effects of DHA on lymphopoiesis. Thus DHA itself may act on lymphoid progenitor cells and/or the microenvironment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett-Connor E., Khaw K. T., Yen S. S. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986 Dec 11;315(24):1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- Beamer W. G., Shultz K. L., Tennent B. J. Induction of ovarian granulosa cell tumors in SWXJ-9 mice with dehydroepiandrosterone. Cancer Res. 1988 May 15;48(10):2788–2792. [PubMed] [Google Scholar]
- Bennett M. Graft-versus-host reactions in mice. I. Kinetic and immunogenetic studies of alloantigen-sensitive units of lymphoid tissue. Transplantation. 1971 Feb;11(2):158–169. [PubMed] [Google Scholar]
- Bennett M., Uauy R., Grundy S. M. Dietary fatty acid effects on T-cell-mediated immunity in mice infected with mycoplasma pulmonis or given carcinogens by injection. Am J Pathol. 1987 Jan;126(1):103–113. [PMC free article] [PubMed] [Google Scholar]
- Brownsey B., Cameron E. H., Griffiths K., Gleave E. N., Forrest A. P., Campbell H. Plasma dehydroepiandrosterone sulphate levels in patients with benign and malignant breast disease. Eur J Cancer. 1972 Feb;8(1):131–137. doi: 10.1016/0014-2964(72)90094-1. [DOI] [PubMed] [Google Scholar]
- Cleary M. P., Seidenstat R., Tannen R. H., Schwartz A. G. The effect of dehydroepiandrosterone on adipose tissue cellularity in mice. Proc Soc Exp Biol Med. 1982 Dec;171(3):276–284. doi: 10.3181/00379727-171-41511. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Leiter E. H., Applezweig N. Therapeutic effects of dehydroepiandrosterone metabolites in diabetes mutant mice (C57BL/KsJ-db/db). Endocrinology. 1984 Jul;115(1):239–243. doi: 10.1210/endo-115-1-239. [DOI] [PubMed] [Google Scholar]
- Curry J. L., Trentin J. J. Hemopoietic spleen colony studies. I. Growth and differentiation. Dev Biol. 1967 May;15(5):395–413. doi: 10.1016/0012-1606(67)90034-6. [DOI] [PubMed] [Google Scholar]
- Garcea R., Daino L., Pascale R., Frassetto S., Cozzolino P., Ruggiu M. E., Feo F. Inhibition by dehydroepiandrosterone of liver preneoplastic foci formation in rats after initiation-selection in experimental carcinogenesis. Toxicol Pathol. 1987;15(2):164–169. doi: 10.1177/019262338701500206. [DOI] [PubMed] [Google Scholar]
- Hackett J., Jr, Bennett M., Kumar V. Origin and differentiation of natural killer cells. I. Characteristics of a transplantable NK cell precursor. J Immunol. 1985 Jun;134(6):3731–3738. [PubMed] [Google Scholar]
- Henderson E., Schwartz A., Pashko L., Abou-Gharbia M., Swern D. Dehydroepiandrosterone and 16 alpha-bromo-epiandrosterone: inhibitors of Epstein-Barr virus-induced transformation of human lymphocytes. Carcinogenesis. 1981;2(7):683–686. doi: 10.1093/carcin/2.7.683. [DOI] [PubMed] [Google Scholar]
- Leiter E. H., Beamer W. G., Coleman D. L., Longcope C. Androgenic and estrogenic metabolites in serum of mice fed dehydroepiandrosterone: relationship to antihyperglycemic effects. Metabolism. 1987 Sep;36(9):863–869. doi: 10.1016/0026-0495(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Loria R. M., Inge T. H., Cook S. S., Szakal A. K., Regelson W. Protection against acute lethal viral infections with the native steroid dehydroepiandrosterone (DHEA). J Med Virol. 1988 Nov;26(3):301–314. doi: 10.1002/jmv.1890260310. [DOI] [PubMed] [Google Scholar]
- Lucas J. A., Ahmed S. A., Casey M. L., MacDonald P. C. Prevention of autoantibody formation and prolonged survival in New Zealand black/New Zealand white F1 mice fed dehydroisoandrosterone. J Clin Invest. 1985 Jun;75(6):2091–2093. doi: 10.1172/JCI111929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Banks J. INHIBITION OF MAMMALIAN GLUCOSE-6-PHOSPHATE DEHYDROGENASE BY STEROIDS. Proc Natl Acad Sci U S A. 1960 Apr;46(4):447–452. doi: 10.1073/pnas.46.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M. C., Tharp M. D., Sullivan T. J., Tigelaar R. E. Contact hypersensitivity reactions to dinitrofluorobenzene mediated by monoclonal IgE anti-DNP antibodies. J Immunol. 1983 Sep;131(3):1096–1102. [PubMed] [Google Scholar]
- Roubinian J. R., Talal N., Greenspan J. S., Goodman J. R., Siiteri P. K. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies, and glomerulonephritis in NZB/NZW F1 mice. J Exp Med. 1978 Jun 1;147(6):1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. G. Inhibition of spontaneous breast cancer formation in female C3H(Avy/a) mice by long-term treatment with dehydroepiandrosterone. Cancer Res. 1979 Mar;39(3):1129–1132. [PubMed] [Google Scholar]
- Shantz L. M., Talalay P., Gordon G. B. Mechanism of inhibition of growth of 3T3-L1 fibroblasts and their differentiation to adipocytes by dehydroepiandrosterone and related steroids: role of glucose-6-phosphate dehydrogenase. Proc Natl Acad Sci U S A. 1989 May;86(10):3852–3856. doi: 10.1073/pnas.86.10.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R. H., Kristie J. A., Cheney K. E., Walford R. L. Influence of controlled dietary restriction on immunologic function and aging. Fed Proc. 1979 May;38(6):2007–2016. [PubMed] [Google Scholar]
- Wolf N. S., Trentin J. J. Hemopoietic colony studies. V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968 Jan 1;127(1):205–214. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]





