Abstract
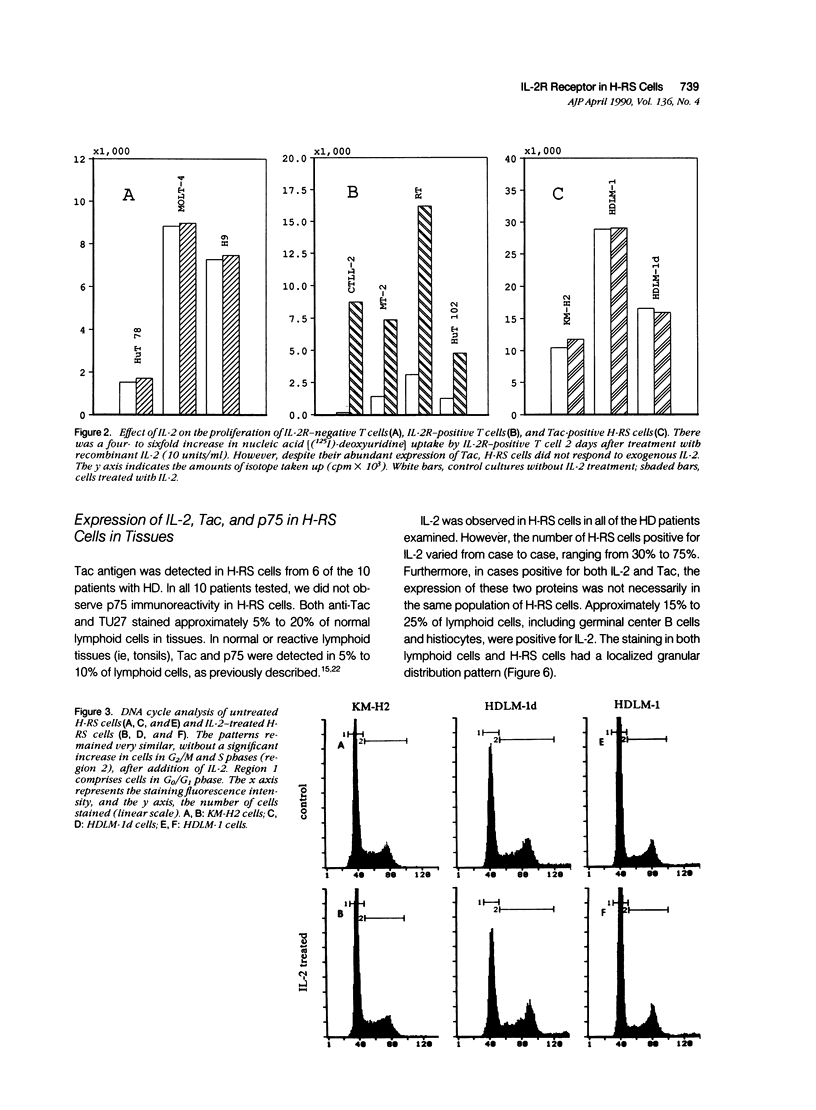
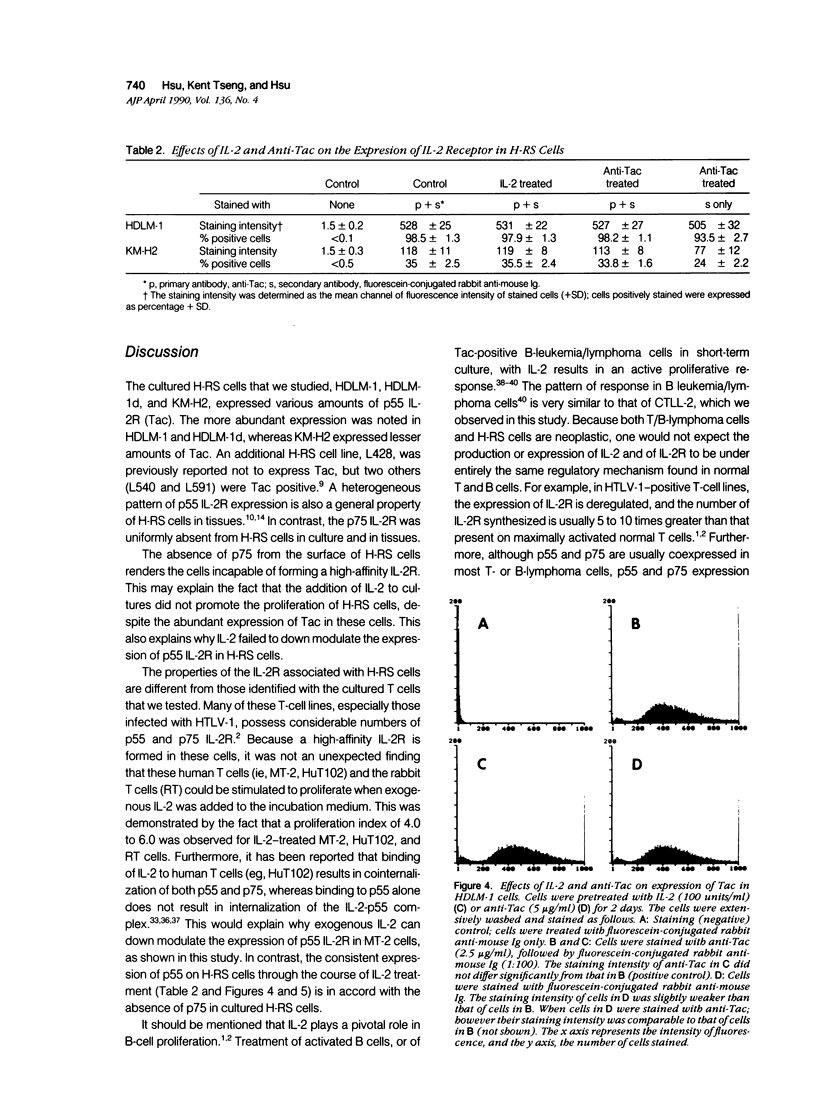
The authors studied the secretion of interleukin-2 (IL-2), the expression of interleukin-2 receptors (IL-2R; p55/Tac and p75), and the response to exogenous IL-2 by cultured Hodgkin's Reed-Sternberg cells (cell lines HDLM-1, HDLM-1d, and KM-H2) and T cells (H9, HuT78, HuT102, MOLT-4, and MT-2). All of these cells did not produce IL-2 or produced it in undetectable amounts, and their growth was not affected by the addition of anti-IL-2 or anti-IL-2R antibodies. This indicates that H-RS cells in long-term culture, as well as T cells, can grow independently of IL-2. The three H-RS cell lines, as well as two of the T-cell lines (HuT102 and MT-2), expressed Tac, whereas the other three T-cell lines were Tac negative. Expression of p75 was noted in the two Tac-positive T-cell lines, but not in cultured H-RS cells. The expression of Tac and p75 in HuT102 and MT-2 cells correlated well with their capacity to proliferate on treatment with exogenous IL-2. On IL-2 treatment, nucleic-acid uptake in Tac/p75-positive T cells increased approximately four- to sixfold, whereas the Tac/p75-negative T cells did not show increased proliferation. Unlike the T cells, the Tac-positive H-RS cells did not respond to IL-2. The lack of a proliferative response to IL-2 appears to be related to the absence of p75 in H-RS cells. A similar pattern (Tac positivity and p75 negativity) was noted in H-RS cells in lymph nodes involved by Hodgkin's disease. Thus the exogenous IL-2 released by surrounding T lymphocytes may not cause the proliferative activity of H-RS cells because of the lack of high-affinity IL-2 receptors in the latter cells. In contrast to H-RS cells in culture, H-RS cells in tissues were stained by a specific anti-IL-2 monoclonal antibody. This indicates that the expression of IL-2 or an IL-2-like substance by H-RS cells in tissues may be responsible, in part, for the great increase in the number of reactive T lymphocytes in tissues involved by Hodgkin's disease.
Full text
PDF



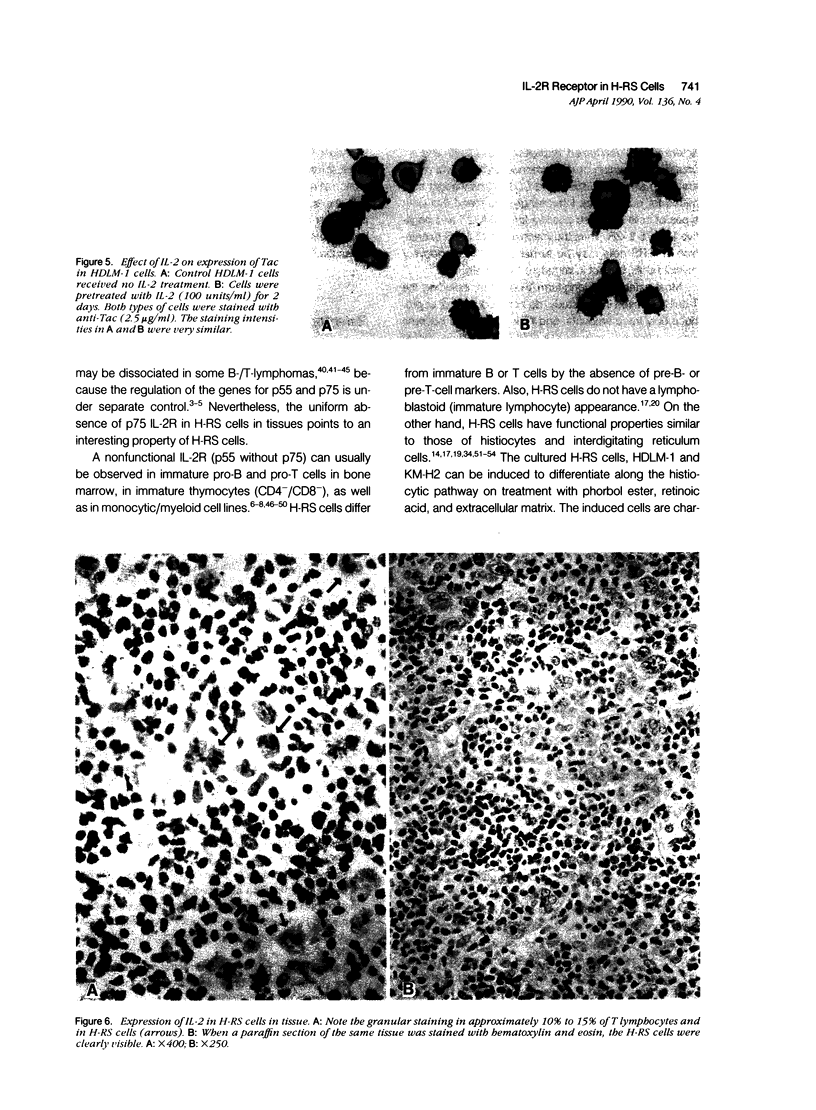



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima N., Daitoku Y., Ohgaki S., Fukumori J., Tanaka H., Yamamoto Y., Fujimoto K., Onoue K. Autocrine growth of interleukin 2-producing leukemic cells in a patient with adult T cell leukemia. Blood. 1986 Sep;68(3):779–782. [PubMed] [Google Scholar]
- Benjamin D., Hartmann D. P., Bazar L. S., Jacobson R. J., Gilmore M. S. Human B cell lines can be triggered to secrete an interleukin 2-like molecule. Cell Immunol. 1989 Jun;121(1):30–48. doi: 10.1016/0008-8749(89)90003-8. [DOI] [PubMed] [Google Scholar]
- Debatin K. M., Woodroofe C., Lahm H., Fischer J., Falk W., Brandeis W. E., Krammer P. H. Lack of interleukin-2 (IL-2) dependent growth of TAC positive T-ALL/NHL cells is due to the expression of only low affinity receptors for IL-2. Leukemia. 1989 Aug;3(8):566–571. [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Lok M. S., Diehl V., Minowada J. Hodgkin's disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res. 1986;10(5):487–500. doi: 10.1016/0145-2126(86)90084-6. [DOI] [PubMed] [Google Scholar]
- Duprez V., Cornet V., Dautry-Varsat A. Down-regulation of high affinity interleukin 2 receptors in a human tumor T cell line. Interleukin 2 increases the rate of surface receptor decay. J Biol Chem. 1988 Sep 15;263(26):12860–12865. [PubMed] [Google Scholar]
- Erber W. N., Mason D. Y. Expression of the interleukin-2 receptor (Tac antigen/CD25) in hematologic neoplasms. Am J Clin Pathol. 1988 May;89(5):645–648. doi: 10.1093/ajcp/89.5.645. [DOI] [PubMed] [Google Scholar]
- Faure G. C., Bene M. C., Bolle-Chanal M. H. Expression of CD 25 (Tac antigen) in lymphoid leukemias and non-Hodgkin lymphomas. Eur J Haematol. 1987 Jan;38(1):26–30. doi: 10.1111/j.1600-0609.1987.tb01419.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Krammer P. H., Diamantstein T., Uhr J. W., Vitetta E. S. B cell-stimulatory factor 1 (BSF-1) promotes growth of helper T cell lines. J Exp Med. 1986 Aug 1;164(2):580–593. doi: 10.1084/jem.164.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. R., Ju G., Greene W. C. Co-internalization of the p55 and p70 subunits of the high-affinity human interleukin 2 receptor. Evidence for a stable ternary receptor complex. J Exp Med. 1988 Nov 1;168(5):1923–1928. doi: 10.1084/jem.168.5.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Takeshita T., Sugamura K. Adenosine 3',5'-cyclic monophosphate (cAMP) inhibits phorbol ester-induced growth of an IL-2-dependent T cell line. FEBS Lett. 1988 Nov 7;239(2):165–168. doi: 10.1016/0014-5793(88)80909-8. [DOI] [PubMed] [Google Scholar]
- Greene W. C. An overview of the human interleukin-2 receptor: molecular, biochemical, and functional properties. Cancer Invest. 1987;5(4):369–376. [PubMed] [Google Scholar]
- Hardy K. J., Manger B., Newton M., Stobo J. D. Molecular events involved in regulating human interferon-gamma gene expression during T cell activation. J Immunol. 1987 Apr 1;138(7):2353–2358. [PubMed] [Google Scholar]
- Herrmann F., Cannistra S. A., Levine H., Griffin J. D. Expression of interleukin 2 receptors and binding of interleukin 2 by gamma interferon-induced human leukemic and normal monocytic cells. J Exp Med. 1985 Sep 1;162(3):1111–1116. doi: 10.1084/jem.162.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. L., Hsu S. M. Production of tumor necrosis factor-alpha and lymphotoxin by cells of Hodgkin's neoplastic cell lines HDLM-1 and KM-H2. Am J Pathol. 1989 Oct;135(4):735–745. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Ho Y. S., Hsu P. L. Effect of monoclonal antibodies anti-2H9, anti-IRac, and anti-HeFi-1 on the surface antigens of Reed-Sternberg cells. J Natl Cancer Inst. 1987 Nov;79(5):1091–1099. [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L., Lo S. S., Wu K. K. Expression of prostaglandin H synthase (cyclooxygenase) in Hodgkin's mononuclear and Reed-Sternberg cells. Functional resemblance between H-RS cells and histiocytes or interdigitating reticulum cells. Am J Pathol. 1988 Oct;133(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Phenotypes and phorbol ester-induced differentiation of human histiocytic lymphoma cell lines (U-937 and SU-DHL-1) and Reed-Sternberg cells. Am J Pathol. 1986 Feb;122(2):223–230. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of B-lymphocytes. 1. Identification with monoclonal antibodies in normal lymphoid tissues. Am J Pathol. 1984 Mar;114(3):387–395. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of T lymphocytes in thymus and peripheral lymphoid tissues. Am J Pathol. 1985 Oct;121(1):69–78. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Krupen K., Lachman L. B. Heterogeneity of interleukin 1 production in cultured Reed-Sternberg cell lines HDLM-1, HDLM-1d, and KM-H2. Am J Pathol. 1989 Jul;135(1):33–38. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Hairy cell leukemia: a B cell neoplasm with a unique antigenic phenotype. Am J Clin Pathol. 1983 Oct;80(4):421–428. doi: 10.1093/ajcp/80.4.421. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. Expression of interleukin-1 in Reed-Sternberg cells and neoplastic cells from true histiocytic malignancies. Am J Pathol. 1986 Nov;125(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Hsu P. L., Lok M. S. Extracellular matrix does not induce the proliferation, but promotes the differentiation, of Hodgkin's cell line HDLM-1. Am J Pathol. 1987 Apr;127(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Kamesaki H., Fukuhara S., Tatsumi E., Uchino H., Yamabe H., Miwa H., Shirakawa S., Hatanaka M., Honjo T. Cytochemical, immunologic, chromosomal, and molecular genetic analysis of a novel cell line derived from Hodgkin's disease. Blood. 1986 Jul;68(1):285–292. [PubMed] [Google Scholar]
- Kimura Y., Toki H., Okabe K., Suzuki K., Hayakawa T., Miyamoto K. Establishment and characterization of a monocytic cell line which expresses the interleukin-2 receptor. Jpn J Cancer Res. 1986 Sep;77(9):862–865. [PubMed] [Google Scholar]
- Kimura Y., Toki H., Okabe K., Suzuki K., Hayakawa T., Miyamoto K. Establishment and characterization of a monocytic cell line which expresses the interleukin-2 receptor. Jpn J Cancer Res. 1986 Sep;77(9):862–865. [PubMed] [Google Scholar]
- Kondo S., Kinoshita M., Shimizu A., Saito Y., Konishi M., Sabe H., Honjo T. Expression and functional characterization of artificial mutants of interleukin-2 receptor. Nature. 1987 May 7;327(6117):64–67. doi: 10.1038/327064a0. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Yodoi J., Nikaido T., Tagaya Y., Taniguchi Y., Honjo T., Yahara I. Expression of interleukin 2 receptors on interleukin 3-dependent cell lines. J Immunol. 1986 Feb 1;136(3):984–987. [PubMed] [Google Scholar]
- Lantz O., Grillot-Courvalin C., Schmitt C., Fermand J. P., Brouet J. C. Interleukin 2-induced proliferation of leukemic human B cells. J Exp Med. 1985 May 1;161(5):1225–1230. doi: 10.1084/jem.161.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Howe R. C., Ceredig R., MacDonald H. R. Functional status of interleukin 2 receptors expressed by immature (Lyt-2-/L3T4-) thymocytes. J Immunol. 1986 Oct 15;137(8):2579–2584. [PubMed] [Google Scholar]
- Maeda M., Noma T., Hama K., Honjo T. Application of a human T cell line derived from a Sézary syndrome patient for human interleukin 4 assay. Immunol Lett. 1988 Aug;18(4):247–253. doi: 10.1016/0165-2478(88)90170-8. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Bierend K. F., Mühlradt P. F. Induction of lymphokine synthesis in peripheral blood mononuclear cells with phorbol ester and calcium ionophore allows precise measurement of individual variations in capacity to produce IL 2. Lymphokine Res. 1986;5 (Suppl 1):S67–S73. [PubMed] [Google Scholar]
- Panayotides P., Lenkei R., Porwit A., Reizenstein P. Malignant B-cells have receptors for and respond to interleukin-2. Med Oncol Tumor Pharmacother. 1986;3(3-4):255–263. doi: 10.1007/BF02935002. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum J., De Smedt M. Differentiation of thymocytes in fetal organ culture: lack of evidence for the functional role of the interleukin 2 receptor expressed by prothymocytes. Eur J Immunol. 1988 May;18(5):795–799. doi: 10.1002/eji.1830180521. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Sabe H., Suzuki N., Kondo S., Ogura T., Shimizu A., Honjo T. A larger number of L chains (Tac) enhance the association rate of interleukin 2 to the high affinity site of the interleukin 2 receptor. J Exp Med. 1988 Nov 1;168(5):1563–1572. doi: 10.1084/jem.168.5.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarting R., Gerdes J., Ziegler A., Stein H. Immunoprecipitation of the interleukin-2 receptor from Hodgkin's disease derived cell lines by monoclonal antibodies. Hematol Oncol. 1987 Jan-Mar;5(1):57–64. doi: 10.1002/hon.2900050107. [DOI] [PubMed] [Google Scholar]
- Sheibani K., Winberg C. D., van de Velde S., Blayney D. W., Rappaport H. Distribution of lymphocytes with interleukin-2 receptors (TAC antigens) in reactive lymphoproliferative processes, Hodgkin's disease, and non-Hodgkin's lymphomas. An immunohistologic study of 300 cases. Am J Pathol. 1987 Apr;127(1):27–37. [PMC free article] [PubMed] [Google Scholar]
- Sideras P., Palacios R. Bone marrow pro-T and pro-B lymphocyte clones express functional receptors for interleukin (IL) 3 and IL 4/BSF-1 and nonfunctional receptors for IL 2. Eur J Immunol. 1987 Feb;17(2):217–221. doi: 10.1002/eji.1830170211. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Favata M. F., Oroszlan S. Production and characterization of monoclonal antibodies to human interleukin 2: strategy and tactics. J Immunol. 1983 Oct;131(4):1808–1815. [PubMed] [Google Scholar]
- Tagawa S., Hatakeyama M., Shibano M., Taniguchi T., Kitani T. The expression of the p75 subunit of interleukin 2 receptor in Tac negative leukemic cells of two patients with large granular lymphocytic leukemia. Blood. 1988 Apr;71(4):1161–1164. [PubMed] [Google Scholar]
- Takeshita T., Goto Y., Tada K., Nagata K., Asao H., Sugamura K. Monoclonal antibody defining a molecule possibly identical to the p75 subunit of interleukin 2 receptor. J Exp Med. 1989 Apr 1;169(4):1323–1332. doi: 10.1084/jem.169.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tentori L., Longo D. L., Zuñiga-Pflucker J. C., Wing C., Kruisbeek A. M. Essential role of the interleukin 2-interleukin 2 receptor pathway in thymocyte maturation in vivo. J Exp Med. 1988 Nov 1;168(5):1741–1747. doi: 10.1084/jem.168.5.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touw I., Löwenberg B. Interleukin 2 stimulates chronic lymphocytic leukemia colony formation in vitro. Blood. 1985 Jul;66(1):237–240. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Umadome H., Uchiyama T., Onishi R., Hori T., Uchino H., Nesumi N. Leukemic cells from a chronic T-lymphocytic leukemia patient proliferated in response to both interleukin-2 and interleukin-4 without prior stimulation and produced interleukin-2 mRNA with stimulation. Blood. 1988 Oct;72(4):1177–1181. [PubMed] [Google Scholar]
- Waldmann T. A. The role of the multichain IL-2 receptor complex in the control of normal and malignant T-cell proliferation. Environ Health Perspect. 1987 Nov;75:11–15. doi: 10.1289/ehp.877511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]