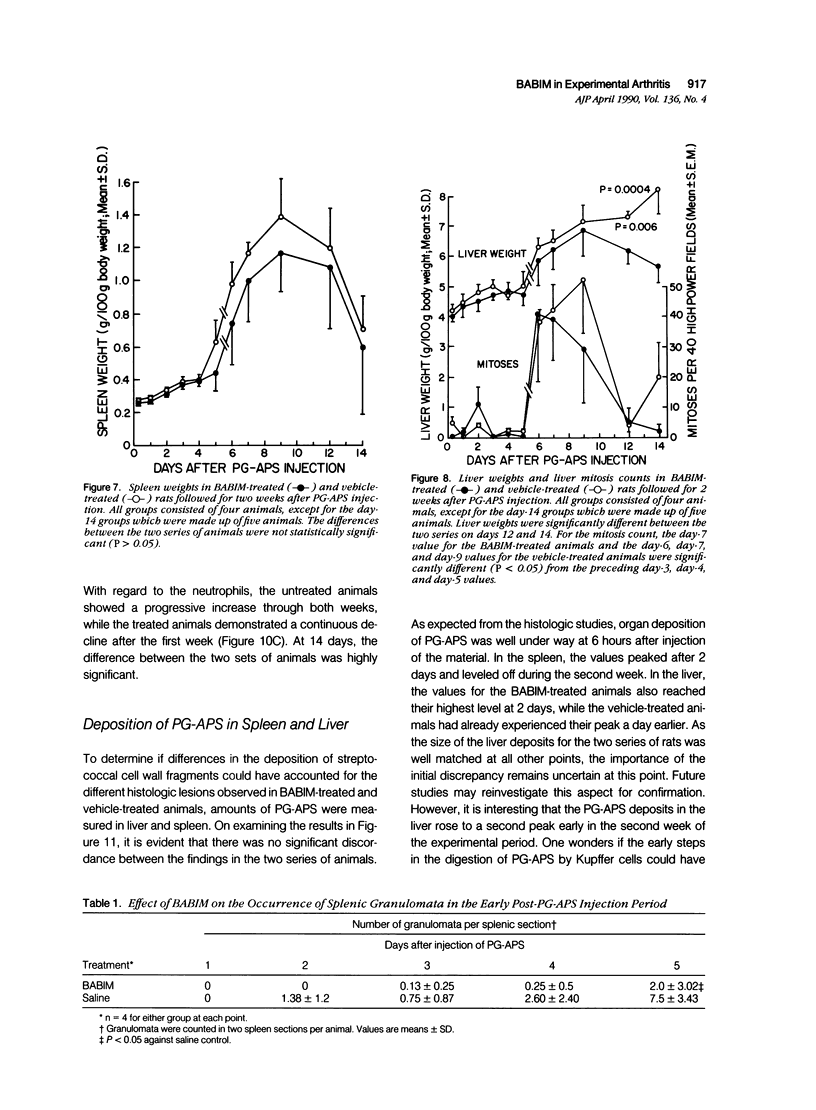
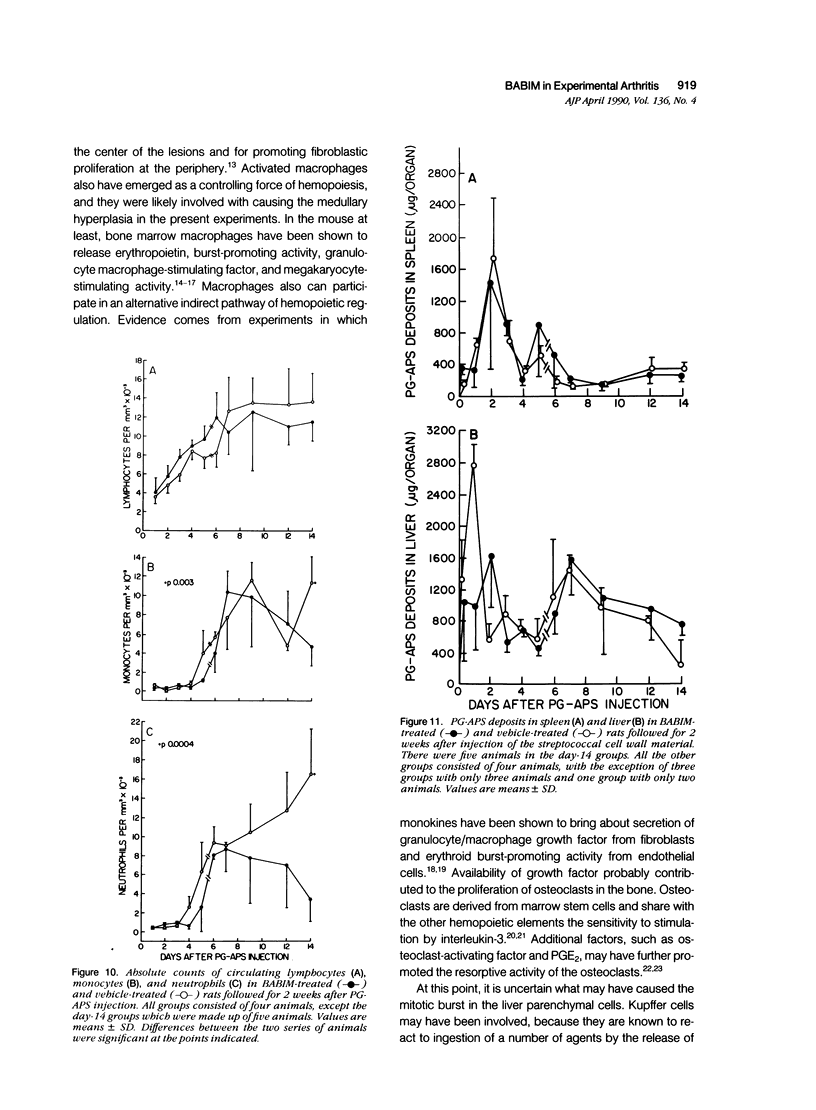
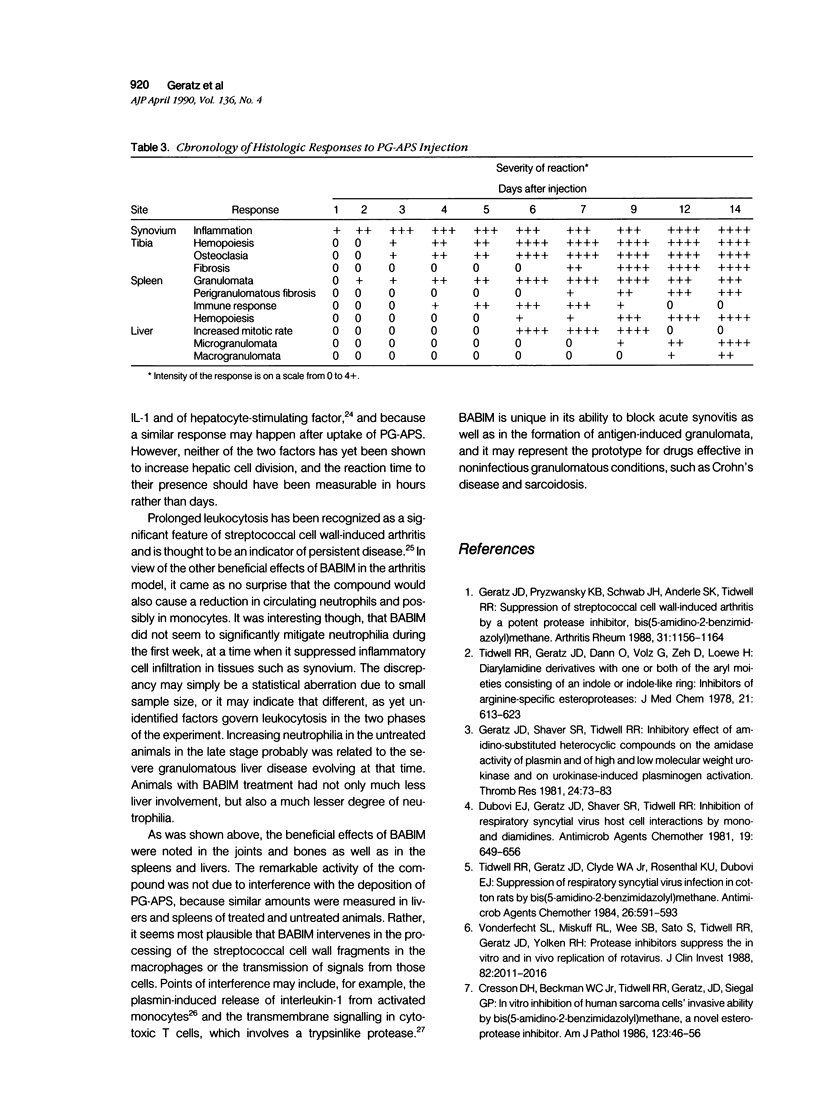
Abstract
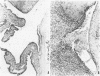
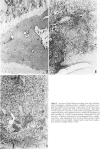
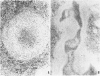
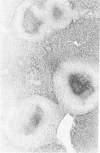
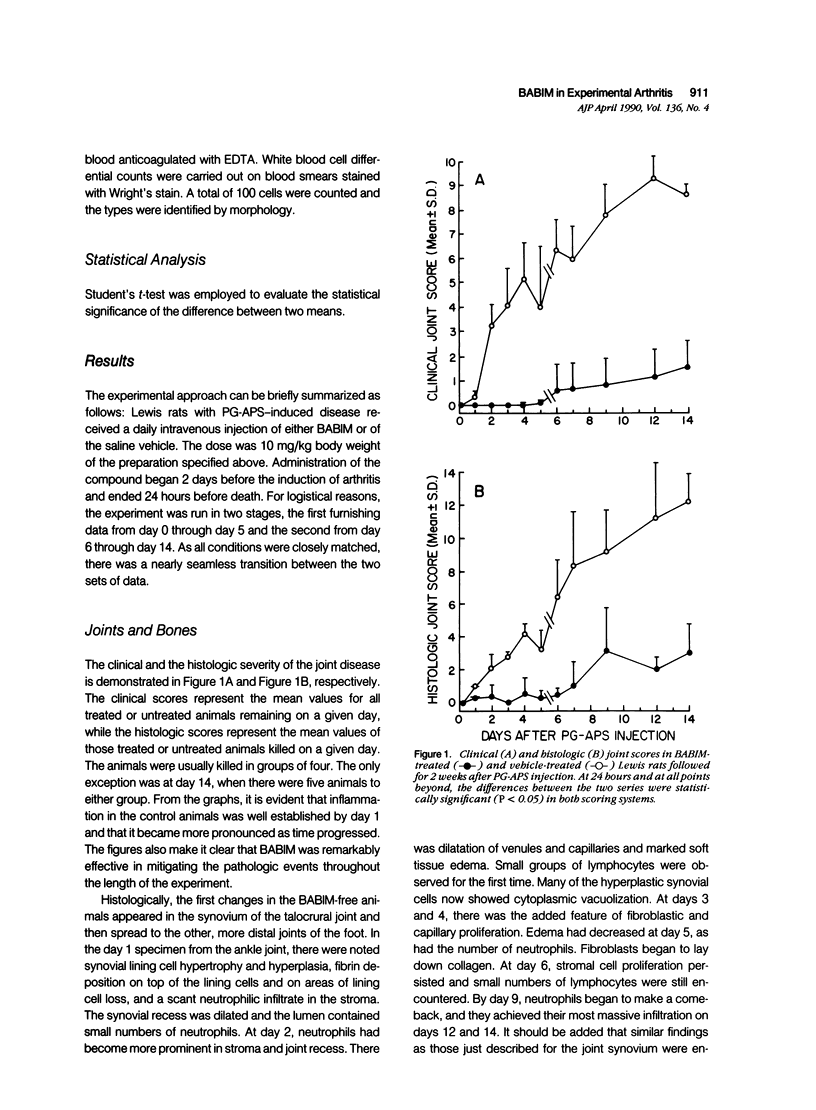
This report builds on the authors' earlier discovery of bis(5-amidino-2-benzimidazolyl)methane (BABIM) as a strong suppressive agent for streptococcal cell wall fragment-induced arthritis in the Lewis rat. As a synthetic inhibitor of trypsinlike proteases, BABIM opens up a new route to the control of inflammatory joint disease. To gain a deeper insight into the function of the compound, the authors have now studied its influence on the sequential development of the joint changes and the associated lesions in spleen and liver. Bis(5-amidino-2-benzimidazolyl)methane is shown to block acute synovitis, to retard and reduce granuloma formation in spleen and liver, to decrease neutrophilic leukocytosis, and to diminish hemopoietic hyperplasia in the bone, and thus also to mitigate the distinctive osteoclastic and chondroclastic events. The compound does not interfere with the splenic immune response, the temporary rise in hepatocytic mitotic activity, or the organ deposition of streptococcal cell walls.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
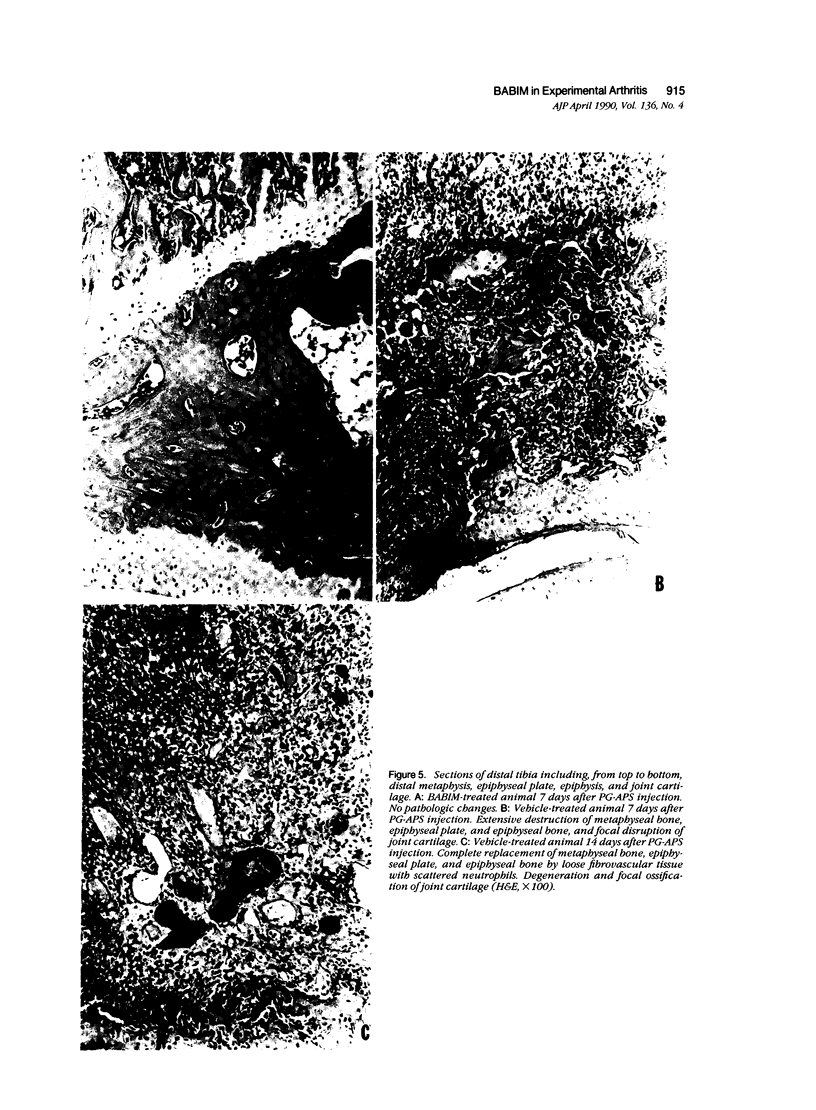
- Anderle S. K., Greenblatt J. J., Cromartie W. J., Clark R., Schwab J. H. Modulation of the susceptibility of inbred and outbred rats to arthritis induced by cell walls of group A streptococci. Infect Immun. 1979 Aug;25(2):484–490. doi: 10.1128/iai.25.2.484-490.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby G. C., Jr, McCall E., Layman D. L. Regulation of colony-stimulating activity production. Interactions of fibroblasts, mononuclear phagocytes, and lactoferrin. J Clin Invest. 1983 Feb;71(2):340–344. doi: 10.1172/JCI110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresson D. H., Beckman W. C., Jr, Tidwell R. R., Geratz J. D., Siegal G. P. In vitro inhibition of human sarcoma cells' invasive ability by bis(5-amidino-2-benzimidazolyl)methane--a novel esteroprotease inhibitor. Am J Pathol. 1986 Apr;123(1):46–56. [PMC free article] [PubMed] [Google Scholar]
- Dalldorf F. G., Cromartie W. J., Anderle S. K., Clark R. L., Schwab J. H. The relation of experimental arthritis to the distribution of streptococcal cell wall fragments. Am J Pathol. 1980 Aug;100(2):383–402. [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Geratz J. D., Shaver S. R., Tidwell R. R. Inhibition of respiratory syncytial virus-host cell interactions by mono- and diamidines. Antimicrob Agents Chemother. 1981 Apr;19(4):649–656. doi: 10.1128/aac.19.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
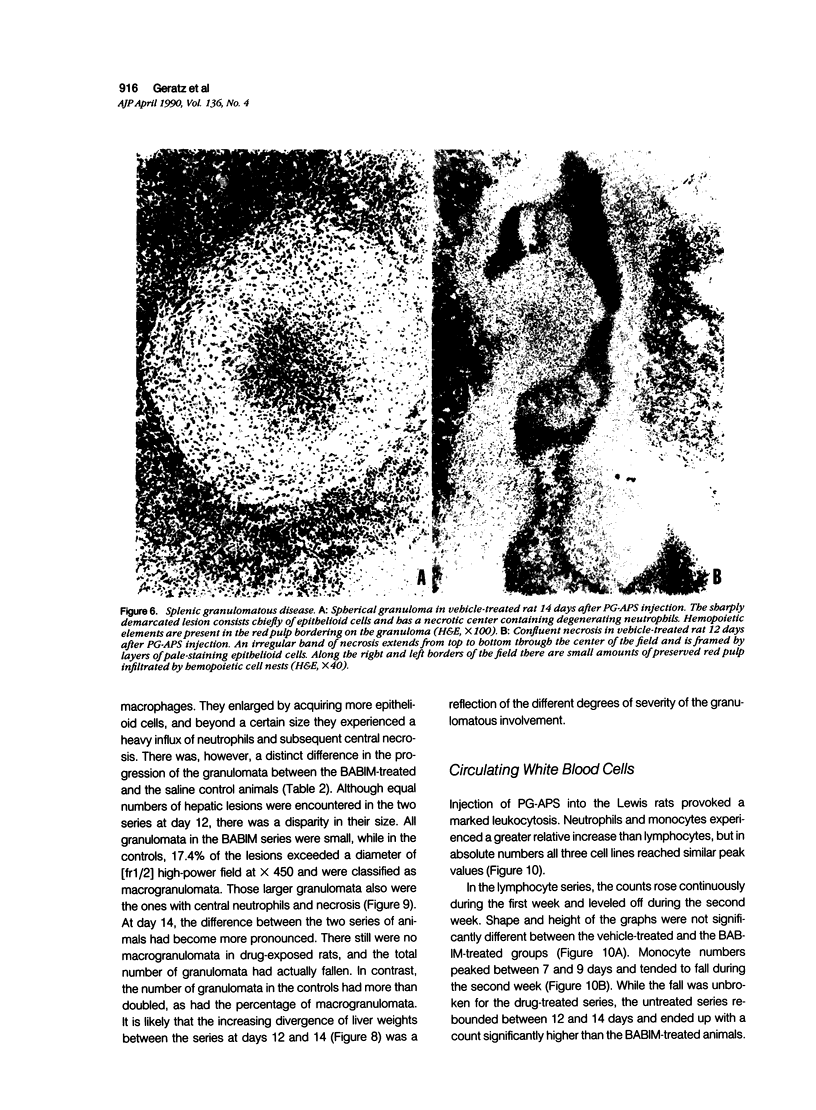
- Eisenberg R. A., Schwab J. H. Arthropathic group A streptococcal cell walls require specific antibody for activation of human complement by both the classical and alternative pathways. Infect Immun. 1986 Aug;53(2):324–330. doi: 10.1128/iai.53.2.324-330.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L., Cohen C. M., Dainiak N. In vitro release of physically separable factors from monocytes that exert opposing effects on erythropoiesis. Blood. 1986 May;67(5):1454–1459. [PubMed] [Google Scholar]
- Fuller K., Chambers T. J. Generation of osteoclasts in cultures of rabbit bone marrow and spleen cells. J Cell Physiol. 1987 Sep;132(3):441–452. doi: 10.1002/jcp.1041320306. [DOI] [PubMed] [Google Scholar]
- Geratz J. D., Pryzwansky K. B., Schwab J. H., Anderle S. K., Tidwell R. R. Suppression of streptococcal cell wall-induced arthritis by a potent protease inhibitor, bis(5-amidino-2-benzimidazolyl)methane. Arthritis Rheum. 1988 Sep;31(9):1156–1164. doi: 10.1002/art.1780310911. [DOI] [PubMed] [Google Scholar]
- Geratz J. D., Shaver S. R., Tidwell R. R. Inhibitory effect of amidino-substituted heterocyclic compounds on the amidase activity of plasmin and of high and low molecular weight urokinase and on urokinase-induced plasminogen activation. Thromb Res. 1981 Oct 1;24(1-2):73–83. doi: 10.1016/0049-3848(81)90033-5. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Rich I. N. A role for the macrophage in normal hemopoiesis. I. Functional capacity of bone-marrow-derived macrophages to release hemopoietic growth factors. Exp Hematol. 1986 Sep;14(8):738–745. [PubMed] [Google Scholar]
- Schelling S. H., Wolfe H. J., Tashjian A. H., Jr Role of the osteoclast in prostaglandin E2-stimulated bone resorption: a correlative morphometric and biochemical analysis. Lab Invest. 1980 Mar;42(3):290–295. [PubMed] [Google Scholar]
- Scheven B. A., Visser J. W., Nijweide P. J. In vitro osteoclast generation from different bone marrow fractions, including a highly enriched haematopoietic stem cell population. Nature. 1986 May 1;321(6065):79–81. doi: 10.1038/321079a0. [DOI] [PubMed] [Google Scholar]
- Shirahama M., Ishibashi H., Tsuchiya Y., Kurokawa S., Okumura Y., Niho Y. Kinetics and parameters of the induction of interleukin 1 secretion by rat Kupffer cells. J Clin Lab Immunol. 1988 Nov;27(3):127–132. [PubMed] [Google Scholar]
- Stimpson S. A., Brown R. R., Anderle S. K., Klapper D. G., Clark R. L., Cromartie W. J., Schwab J. H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986 Jan;51(1):240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Clyde W. A., Jr, Rosenthal K. U., Dubovi E. J. Suppression of respiratory syncytial virus infection in cotton rats by bis(5-amidino-2-benzimidazolyl)methane. Antimicrob Agents Chemother. 1984 Oct;26(4):591–593. doi: 10.1128/aac.26.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell R. R., Geratz J. D., Dann O., Volz G., Zeh D., Loewe H. Diarylamidine derivatives with one or both of the aryl moieties consisting of an indole or indole-like ring. Inhibitors of arginine-specific esteroproteases. J Med Chem. 1978 Jul;21(7):613–623. doi: 10.1021/jm00205a005. [DOI] [PubMed] [Google Scholar]
- Utsunomiya N., Nakanishi M. A serine protease triggers the initial step of transmembrane signalling in cytotoxic T cells. J Biol Chem. 1986 Dec 15;261(35):16514–16517. [PubMed] [Google Scholar]
- Vogt C., Pentz S., Rich I. N. A role for the macrophage in normal hemopoiesis: III. In vitro and in vivo erythropoietin gene expression in macrophages detected by in situ hybridization. Exp Hematol. 1989 Jun;17(5):391–397. [PubMed] [Google Scholar]
- Vonderfecht S. L., Miskuff R. L., Wee S. B., Sato S., Tidwell R. R., Geratz J. D., Yolken R. H. Protease inhibitors suppress the in vitro and in vivo replication of rotavirus. J Clin Invest. 1988 Dec;82(6):2011–2016. doi: 10.1172/JCI113821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Dougherty S., Evequoz V., Pluznik D. H., Wilder R. L., Hand A. R., Wahl L. M. T lymphocyte-dependent evolution of bacterial cell wall-induced hepatic granulomas. J Immunol. 1986 Oct 1;137(7):2199–2209. [PubMed] [Google Scholar]
- Wells A. F., Hightower J. A., Parks C., Kufoy E., Fox A. Systemic injection of group A streptococcal peptidoglycan-polysaccharide complexes elicits persistent neutrophilia and monocytosis associated with polyarthritis in rats. Infect Immun. 1989 Feb;57(2):351–358. doi: 10.1128/iai.57.2.351-358.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N., Jackson H., Ralph P., Nakoinz I. Cell interactions influencing murine marrow megakaryocytes: nature of the potentiator cell in bone marrow. Blood. 1981 Jan;57(1):157–163. [PubMed] [Google Scholar]
- Yoneda T., Mundy G. R. Prostaglandins are necessary for osteoclast-activating factor production by activated peripheral blood leukocytes. J Exp Med. 1979 Jan 1;149(1):279–283. doi: 10.1084/jem.149.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman K. S., Bagby G. C., Jr, McCall E., Sparks B., Wells J., Patel V., Goodrum D. A monokine stimulates production of human erythroid burst-promoting activity by endothelial cells in vitro. J Clin Invest. 1985 Feb;75(2):722–725. doi: 10.1172/JCI111752. [DOI] [PMC free article] [PubMed] [Google Scholar]