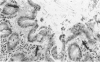Abstract
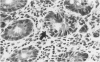
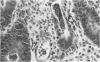
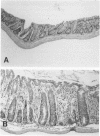
In a study designed to determine which T-cell subsets are involved in the development of murine graft-versus-host disease (GVHD), a prospective histologic analysis of gastrointestinal involvement was performed. In C57BL/6JXDBA/2F1 (B6D2F1) recipients of DBA/2 donor spleen and bone marrow cells, the colonic histologic findings were found to be similar in many respects to the histologic findings reported in human colonic GVHD and were much more severe and diffuse than were the abnormalities of the small intestine. Host irradiation before transplantation was found to play an additive or synergistic role in the development of GVHD. Furthermore the histologic features noted in DBA/2----B6D2F1 murine colonic GVHD suggest that bone marrow and spleen cell transplantation in this strain combination may be a useful model for studying the immunologic mechanisms involved in human inflammatory bowel disease. Thus severe colonic disease noted during the course of DBA/2----B6D2F1 murine GVHD was found to have significant histopathologic similarities to both human GVHD enteropathy and other inflammatory diseases of the human colon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland A., Mowat A. M., Parrott D. M. Augmentation of intestinal and peripheral natural killer cell activity during the graft-versus-host reaction in mice. Transplantation. 1983 Nov;36(5):513–519. doi: 10.1097/00007890-198311000-00009. [DOI] [PubMed] [Google Scholar]
- CONGDON C. C., URSO I. S. Homologous bone marrow in the treatment of radiation injury in mice. Am J Pathol. 1957 Jul-Aug;33(4):749–767. [PMC free article] [PubMed] [Google Scholar]
- Cudkowicz G., Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971 Dec 1;134(6):1513–1528. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Functional properties of lymphocytes isolated from murine small intestinal epithelium. Immunology. 1984 Jul;52(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Reilly R. W., Rosenberg I. H. Small intestinal injury in the graft versus host reaction: an innocent bystander phenomenon. Gastroenterology. 1977 May;72(5 Pt 1):886–889. [PubMed] [Google Scholar]
- Epstein R. J., McDonald G. B., Sale G. E., Shulman H. M., Thomas E. D. The diagnostic accuracy of the rectal biopsy in acute graft-versus-host disease: a prospective study of thirteen patients. Gastroenterology. 1980 Apr;78(4):764–771. [PubMed] [Google Scholar]
- Gale R. P. Graft-versus-host disease. Immunol Rev. 1985 Dec;88:193–214. doi: 10.1111/j.1600-065x.1985.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Vassalli P. Gut injury in mouse graft-versus-host reaction. Study of its occurrence and mechanisms. J Clin Invest. 1986 May;77(5):1584–1595. doi: 10.1172/JCI112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgard H. R. Dissociation of splenomegaly from graft-versus-host disease by host x-irradiation. Transplantation. 1970 Nov;10(5):396–402. doi: 10.1097/00007890-197011000-00006. [DOI] [PubMed] [Google Scholar]
- ILBERY P. L., KOLLER P. C., LOUTIT J. F. Immunological characteristics of radiation chimaeras. J Natl Cancer Inst. 1958 Jun;20(6):1051–1089. doi: 10.1093/jnci/20.6.1051. [DOI] [PubMed] [Google Scholar]
- Kersey J. H., Meuwissen H. J., Good R. A. Graft versus host reactions following transplantation of allogeneic hematopoietic cells. Hum Pathol. 1971 Sep;2(3):389–402. doi: 10.1016/s0046-8177(71)80006-0. [DOI] [PubMed] [Google Scholar]
- Lehnert S., Rybka W. B., Seemayer T. A. Amplification of the graft-versus-host reaction by partial body irradiation. Transplantation. 1986 Jun;41(6):675–679. doi: 10.1097/00007890-198606000-00002. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Ferguson A. Hypersensitivity reactions in the small intestine. III. The effects of allograft rejection and of graft-versus-host disease on epithelial cell kinetics. Cell Tissue Kinet. 1977 Jul;10(4):301–312. [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Martin P. J., Hansen J. A., Storb R., Thomas E. D. Human marrow transplantation: an immunological perspective. Adv Immunol. 1987;40:379–438. doi: 10.1016/s0065-2776(08)60243-6. [DOI] [PubMed] [Google Scholar]
- McDonald G. B., Shulman H. M., Sullivan K. M., Spencer G. D. Intestinal and hepatic complications of human bone marrow transplantation. Part I. Gastroenterology. 1986 Feb;90(2):460–477. doi: 10.1016/0016-5085(86)90949-2. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Borland A., Parrott D. M. Hypersensitivity reactions in the small intestine. VII. Induction of the intestinal phase of murine graft-versus-host-reaction by Lyt 2- T cells activated by I-A alloantigens. Transplantation. 1986 Feb;41(2):192–198. [PubMed] [Google Scholar]
- Mowat A. M. Evidence that Ia+ bone-marrow-derived cells are the stimulus for the intestinal phase of the murine graft-versus-host reaction. Transplantation. 1986 Aug;42(2):141–144. doi: 10.1097/00007890-198608000-00007. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V., Baca M. E. Experimental studies of immunologically mediated enteropathy. III. Severe and progressive enteropathy during a graft-versus-host reaction in athymic mice. Immunology. 1987 Jun;61(2):185–188. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Felstein M. V., Borland A., Parrott D. M. Experimental studies of immunologically mediated enteropathy. Development of cell mediated immunity and intestinal pathology during a graft-versus-host reaction in irradiated mice. Gut. 1988 Jul;29(7):949–956. doi: 10.1136/gut.29.7.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Hypersensitivity reactions in the small intestine. 6. Pathogenesis of the graft-versus-host reaction in the small intestinal mucosa of the mouse. Transplantation. 1981 Sep;32(3):238–243. doi: 10.1097/00007890-198109000-00011. [DOI] [PubMed] [Google Scholar]
- Mowat A. M., Ferguson A. Intraepithelial lymphocyte count and crypt hyperplasia measure the mucosal component of the graft-versus-host reaction in mouse small intestine. Gastroenterology. 1982 Aug;83(2):417–423. [PubMed] [Google Scholar]
- Mowat A. M., MacKenzie S., Baca M. E., Felstein M. V., Parrott D. M. Functional characteristics of intraepithelial lymphocytes from mouse small intestine. II. In vivo and in vitro responses of intraepithelial lymphocytes to mitogenic and allogeneic stimuli. Immunology. 1986 Aug;58(4):627–634. [PMC free article] [PubMed] [Google Scholar]
- NOWELL P. C., COLE L. J. Inhibition of bowel epithelium regeneration by grafted homologous spleen cells in irradiated mice. Transplant Bull. 1961 Jan;27:94–98. doi: 10.1097/00006534-196101000-00032. [DOI] [PubMed] [Google Scholar]
- REILLY R. W., KIRSNER J. B. RUNT INTESTINAL DISEASE. Lab Invest. 1965 Jan;14:102–107. [PubMed] [Google Scholar]
- Rappaport H., Khalil A., Halle-Pannenko O., Pritchard L., Dantchev D., Mathé G. Histopathologic sequence of events in adult mice undergoing lethal graft-versus-host reaction developed across H-2 and/or non-H-2 histocompatibility barriers. Am J Pathol. 1979 Jul;96(1):121–142. [PMC free article] [PubMed] [Google Scholar]
- Sale G. E., Shulman H. M., McDonald G. B., Thomas E. D. Gastrointestinal graft-versus-host disease in man. A clinicopathologic study of the rectal biopsy. Am J Surg Pathol. 1979 Aug;3(4):291–299. doi: 10.1097/00000478-197908000-00001. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Snover D. C., Weisdorf S. A., Vercellotti G. M., Rank B., Hutton S., McGlave P. A histopathologic study of gastric and small intestinal graft-versus-host disease following allogeneic bone marrow transplantation. Hum Pathol. 1985 Apr;16(4):387–392. doi: 10.1016/s0046-8177(85)80232-x. [DOI] [PubMed] [Google Scholar]
- Spencer G. D., Shulman H. M., Myerson D., Thomas E. D., McDonald G. B. Diffuse intestinal ulceration after marrow transplantation: a clinicopathologic study of 13 patients. Hum Pathol. 1986 Jun;17(6):621–633. doi: 10.1016/s0046-8177(86)80135-6. [DOI] [PubMed] [Google Scholar]
- Storb R., Thomas E. D. Graft-versus-host disease in dog and man: the Seattle experience. Immunol Rev. 1985 Dec;88:215–238. doi: 10.1111/j.1600-065x.1985.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Thiele D. L., Charley M. R., Calomeni J. A., Lipsky P. E. Lethal graft-vs-host disease across major histocompatibility barriers: requirement for leucyl-leucine methyl ester sensitive cytotoxic T cells. J Immunol. 1987 Jan 1;138(1):51–57. [PubMed] [Google Scholar]
- Thiele D. L., Eigenbrodt M. L., Bryde S. E., Eigenbrodt E. H., Lipsky P. E. Intestinal graft-versus-host disease is initiated by donor T cells distinct from classic cytotoxic T lymphocytes. J Clin Invest. 1989 Dec;84(6):1947–1956. doi: 10.1172/JCI114383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. Regulation of cellular function by products of lysosomal enzyme activity: elimination of human natural killer cells by a dipeptide methyl ester generated from L-leucine methyl ester by monocytes or polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2468–2472. doi: 10.1073/pnas.82.8.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorning D., Howard J. D. Epithelial denudement in the gastrointestinal tracts of two bone marrow transplant recipients. Hum Pathol. 1986 Jun;17(6):560–566. doi: 10.1016/s0046-8177(86)80127-7. [DOI] [PubMed] [Google Scholar]
- Wagner R., Gabbert H. Morphology and chronology of ischemic mucosal changes in the small intestine. A light and electron microscopic investigation. Klin Wochenschr. 1983 Jun 15;61(12):593–599. doi: 10.1007/BF01487337. [DOI] [PubMed] [Google Scholar]
- Wick M. R., Moore S. B., Gastineau D. A., Hoagland H. C. Immunologic, clinical, and pathologic aspects of human graft-versus-host disease. Mayo Clin Proc. 1983 Sep;58(9):603–612. [PubMed] [Google Scholar]
- Woodruff J. M., Hansen J. A., Good R. A., Santos G. W., Slavin R. E. The pathology of the graft-versus-host reaction (GVHR) in adults receiving bone marrow transplants. Transplant Proc. 1976 Dec;8(4):675–684. [PubMed] [Google Scholar]
- van Bekkum D. W., Knaan S. Role of bacterial microflora in development of intestinal lesions from graft-versus-host reaction. J Natl Cancer Inst. 1977 Mar;58(3):787–790. doi: 10.1093/jnci/58.3.787. [DOI] [PubMed] [Google Scholar]