Abstract
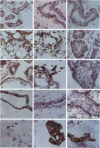
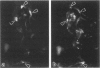
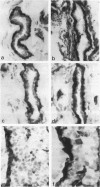
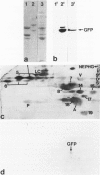
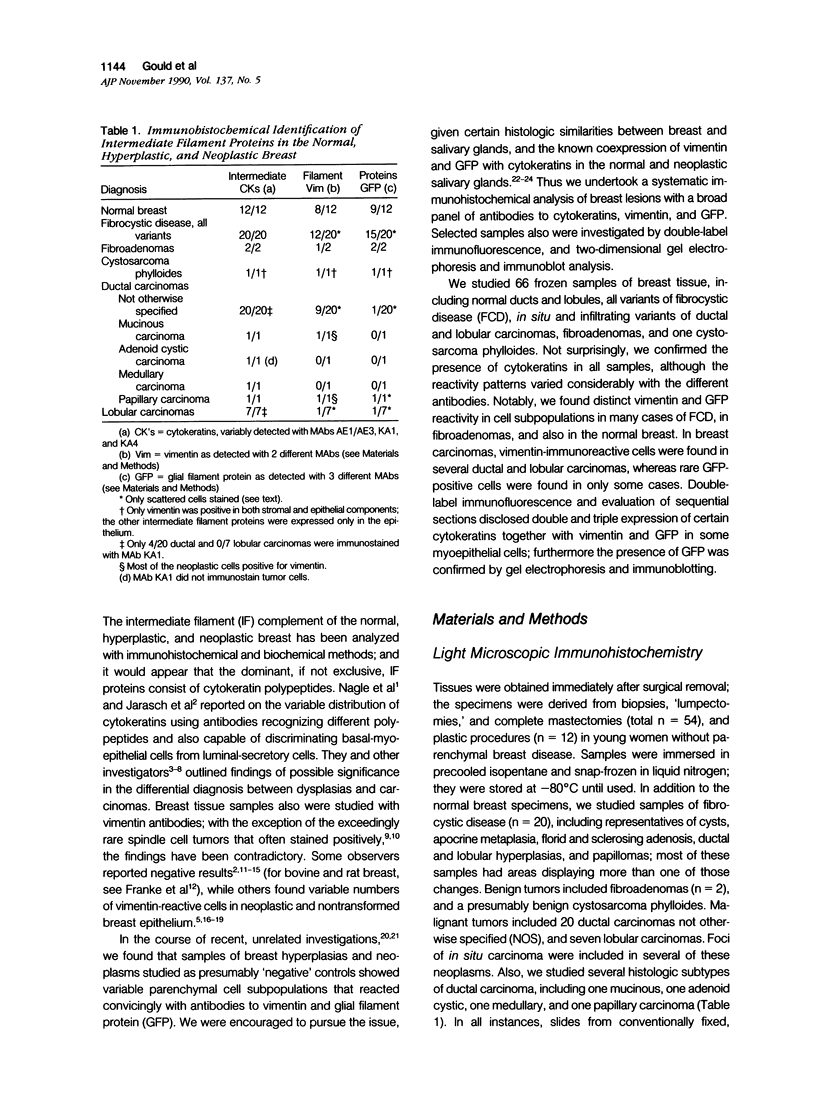
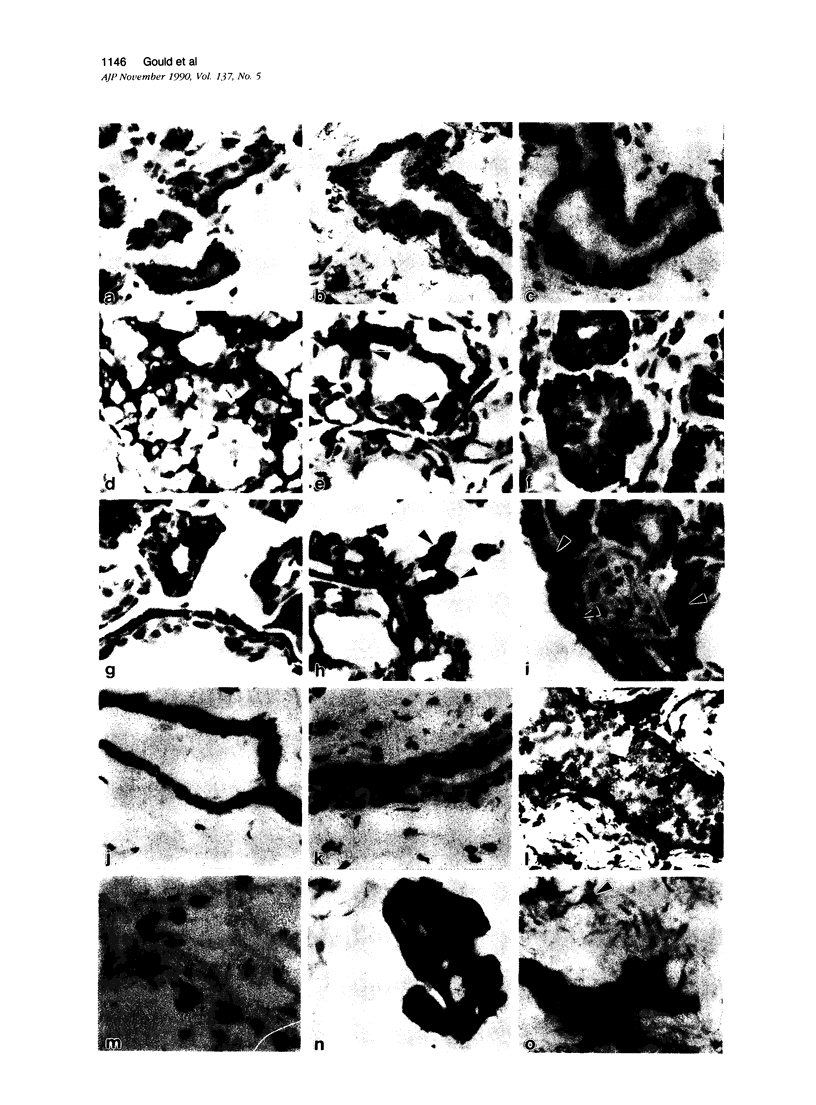
The authors studied by immunohistochemistry the intermediate filament (IF) protein profile of 66 frozen samples of breast tissue, including normal parenchyma, all variants of fibrocystic disease (FCD), fibroadenomas, cystosarcoma phylloides, and ductal and lobular carcinomas. Monoclonal antibodies (MAbs) to cytokeratins included MAb KA 1, which binds to polypeptide 5 in a complex with polypeptide 14 and recognizes preferentially myoepithelial cells; MAb KA4, which binds to polypeptides 14, 15, 16 and 19; individual MAbs to polypeptides 7, 13, and 16, 17, 18, and 19, and the MAb mixture AE1/AE3. The authors also applied three MAbs to vimentin (Vim), and three MAbs to glial filament protein (GFP). Selected samples were studied by double-label immunofluorescence microscopy and by staining sequential sections with some of the said MAbs, an MAb to alpha-smooth muscle actin, and well-characterized polyclonal antibodies for the possible coexpression of diverse types of cytoskeletal proteins. Gel electrophoresis and immunoblot analysis also were performed. All samples reacted for cytokeratins with MAbs AE1/AE3, although the reaction did not involve all cells. Monoclonal antibody KA4 stained preferentially the luminal-secretory cells in the normal breast and in FCD, whereas it stained the vast majority of cells in all carcinomas. Monoclonal antibody KA1 stained preferentially the basal-myoepithelial cells of the normal breast and FCD while staining tumor cell subpopulations in 4 of 31 carcinomas. Vimentin-positive cells were found in 8 of 12 normal breasts and in 12 of 20 FCD; in most cases, Vim-reactive cells appeared to be myoepithelial, but occasional luminal cells were also stained. Variable subpopulations of Vim-positive cells were noted in 9 of 20 ductal and in 1 of 7 lobular carcinomas. Glial filament protein-reactive cells were found in normal breast lobules and ducts and in 15 of 20 cases of FCD; with rare exceptions, GFP-reactivity was restricted to basally located, myoepithelial-appearing cells. Occasional GFP-reactive cells were found in 3 of 31 carcinomas. Evaluation of sequential sections and double-label immunofluorescence microscopy showed the coexpression of certain cytokeratins (possibly including polypeptides 14 and 17) with vimentin and alpha-smooth muscle actin together with GFP in some myoepithelial cells. The presence of GFP in myoepithelial cells was confirmed by gel electrophoresis and immunoblotting. Our results indicate that coexpression of cytokeratin with vimentin and/or GFP is comparatively frequent in normal basal-myoepithelial cells of the breast.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achstätter T., Moll R., Anderson A., Kuhn C., Pitz S., Schwechheimer K., Franke W. W. Expression of glial filament protein (GFP) in nerve sheaths and non-neural cells re-examined using monoclonal antibodies, with special emphasis on the co-expression of GFP and cytokeratins in epithelial cells of human salivary gland and pleomorphic adenomas. Differentiation. 1986;31(3):206–227. doi: 10.1111/j.1432-0436.1986.tb00401.x. [DOI] [PubMed] [Google Scholar]
- Achtstaetter T., Hatzfeld M., Quinlan R. A., Parmelee D. C., Franke W. W. Separation of cytokeratin polypeptides by gel electrophoretic and chromatographic techniques and their identification by immunoblotting. Methods Enzymol. 1986;134:355–371. doi: 10.1016/0076-6879(86)34102-8. [DOI] [PubMed] [Google Scholar]
- Achtstätter T., Fouquet B., Rungger-Brändle E., Franke W. W. Cytokeratin filaments and desmosomes in the epithelioid cells of the perineurial and arachnoidal sheaths of some vertebrate species. Differentiation. 1989 May;40(2):129–149. doi: 10.1111/j.1432-0436.1989.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Achtstätter T., Moll R., Moore B., Franke W. W. Cytokeratin polypeptide patterns of different epithelia of the human male urogenital tract: immunofluorescence and gel electrophoretic studies. J Histochem Cytochem. 1985 May;33(5):415–426. doi: 10.1177/33.5.2580881. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Dirk T., Droese M., Weber K., Osborn M. Keratin polypeptide distribution in benign and malignant breast tumors: subdivision of ductal carcinomas using monoclonal antibodies. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):265–275. doi: 10.1007/BF02899036. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Osborn M., Hölscher A., Schauer A., Weber K. The distribution of keratin type intermediate filaments in human breast cancer. An immunohistological study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(3):277–284. doi: 10.1007/BF02892576. [DOI] [PubMed] [Google Scholar]
- Azumi N., Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987 Sep;88(3):286–296. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- Bignami A., Eng L. F., Dahl D., Uyeda C. T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972 Aug 25;43(2):429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- Blobel G. A., Gould V. E., Moll R., Lee I., Huszar M., Geiger B., Franke W. W. Coexpression of neuroendocrine markers and epithelial cytoskeletal proteins in bronchopulmonary neuroendocrine neoplasms. Lab Invest. 1985 Jan;52(1):39–51. [PubMed] [Google Scholar]
- Bologa L., Cole R., Chiappelli F., Saneto R. P., De Vellis J. Expression of glial fibrillary acidic protein by differentiated astrocytes is regulated by serum antagonistic factors. Brain Res. 1988 Aug 9;457(2):295–302. doi: 10.1016/0006-8993(88)90699-3. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Andreola S., Clemente C., D'Amato L., Rilke F. Vimentin and p53 expression on epidermal growth factor receptor-positive, oestrogen receptor-negative breast carcinomas. Br J Cancer. 1988 Apr;57(4):353–357. doi: 10.1038/bjc.1988.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl D., Bignami A. Intermediate filaments in nervous tissue. Cell Muscle Motil. 1985;6:75–96. doi: 10.1007/978-1-4757-4723-2_4. [DOI] [PubMed] [Google Scholar]
- Dahl D., Rueger D. C., Bignami A., Weber K., Osborn M. Vimentin, the 57 000 molecular weight protein of fibroblast filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol. 1981 Jun;24(2):191–196. [PubMed] [Google Scholar]
- Dairkee S. H., Ljung B. M., Smith H., Hackett A. Immunolocalization of a human basal epithelium specific keratin in benign and malignant breast disease. Breast Cancer Res Treat. 1987 Oct;10(1):11–20. doi: 10.1007/BF01806130. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Deck J. H., Eng L. F., Bigbee J., Woodcock S. M. The role of glial fibrillary acidic protein in the diagnosis of central nervous system tumors. Acta Neuropathol. 1978 Jun 30;42(3):183–190. doi: 10.1007/BF00690355. [DOI] [PubMed] [Google Scholar]
- Doglioni C., Dell'Orto P., Coggi G., Iuzzolino P., Bontempini L., Viale G. Choroid plexus tumors. An immunocytochemical study with particular reference to the coexpression of intermediate filament proteins. Am J Pathol. 1987 Jun;127(3):519–529. [PMC free article] [PubMed] [Google Scholar]
- Ellis I. O., Bell J., Ronan J. E., Elston C. W., Blamey R. W. Immunocytochemical investigation of intermediate filament proteins and epithelial membrane antigen in spindle cell tumours of the breast. J Pathol. 1988 Feb;154(2):157–165. doi: 10.1002/path.1711540208. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Grund C., Achtstätter T. Co-expression of cytokeratins and neurofilament proteins in a permanent cell line: cultured rat PC12 cells combine neuronal and epithelial features. J Cell Biol. 1986 Nov;103(5):1933–1943. doi: 10.1083/jcb.103.5.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Moll R. Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation. 1987;36(2):145–163. doi: 10.1111/j.1432-0436.1987.tb00189.x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gard A. L., White F. P., Dutton G. R. Extra-neural glial fibrillary acidic protein (GFAP) immunoreactivity in perisinusoidal stellate cells of rat liver. J Neuroimmunol. 1985 Jun;8(4-6):359–375. doi: 10.1016/s0165-5728(85)80073-4. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Jansson D. S., Molenaar W. M., Rorke L. B., Trojanowski J. Q., Lee V. M., Packer R. J., Franke W. W. Primitive neuroectodermal tumors of the central nervous system. Patterns of expression of neuroendocrine markers, and all classes of intermediate filament proteins. Lab Invest. 1990 Apr;62(4):498–509. [PubMed] [Google Scholar]
- Gould V. E., Jao W., Battifora H. Ultrastructural analysis in the differential diagnosis of breast tumors. The significance of myoepithelial cells, basal lamina, intracytoplasmic lumina and secretory granules. Pathol Res Pract. 1980 May;167(1):45–70. doi: 10.1016/S0344-0338(80)80181-6. [DOI] [PubMed] [Google Scholar]
- Gould V. E., Moll R., Moll I., Lee I., Schwechheimer K., Franke W. W. The intermediate filament complement of the spectrum of nerve sheath neoplasms. Lab Invest. 1986 Oct;55(4):463–474. [PubMed] [Google Scholar]
- Gould V. E., Rorke L. B., Jansson D. S., Molenaar W. M., Trojanowski J. Q., Lee V. M., Packer R. J., Franke W. W. Primitive neuroectodermal tumors of the central nervous system express neuroendocrine markers and may express all classes of intermediate filaments. Hum Pathol. 1990 Mar;21(3):245–252. doi: 10.1016/0046-8177(90)90223-r. [DOI] [PubMed] [Google Scholar]
- Gould V. W., Snyder R. W. Ultrastructural features of papillomatosis and carcinoma of nipple ducts. The significance of myoepithelial cells and basal lamina in benign, "questionable," and malignant lesions. Pathol Annu. 1974;9(0):441–469. [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to human intermediate filament proteins. III. Analysis of tumors. Am J Clin Pathol. 1985 Oct;84(4):413–424. doi: 10.1093/ajcp/84.4.413. [DOI] [PubMed] [Google Scholar]
- Gray M. H., Rosenberg A. E., Dickersin G. R., Bhan A. K. Glial fibrillary acidic protein and keratin expression by benign and malignant nerve sheath tumors. Hum Pathol. 1989 Nov;20(11):1089–1096. doi: 10.1016/0046-8177(89)90228-1. [DOI] [PubMed] [Google Scholar]
- Guelstein V. I., Tchypysheva T. A., Ermilova V. D., Litvinova L. V., Troyanovsky S. M., Bannikov G. A. Monoclonal antibody mapping of keratins 8 and 17 and of vimentin in normal human mammary gland, benign tumors, dysplasias and breast cancer. Int J Cancer. 1988 Aug 15;42(2):147–153. doi: 10.1002/ijc.2910420202. [DOI] [PubMed] [Google Scholar]
- Gustafsson H., Virtanen I., Thornell L. E. Glial fibrillary acidic protein and desmin in salivary neoplasms. Expression of four different types of intermediate filament proteins within the same cell type. Virchows Arch B Cell Pathol Incl Mol Pathol. 1989;57(5):303–313. doi: 10.1007/BF02899095. [DOI] [PubMed] [Google Scholar]
- Hatfield J. S., Skoff R. P., Maisel H., Eng L. Glial fibrillary acidic protein is localized in the lens epithelium. J Cell Biol. 1984 May;98(5):1895–1898. doi: 10.1083/jcb.98.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley H. R., McNulty J. A., Rowden G. Glial fibrillary acidic protein and S-100 protein in pineal supportive cells: an electron microscopic study. Brain Res. 1984 Jun 18;304(1):117–120. doi: 10.1016/0006-8993(84)90866-7. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Huszar M., Gigi-Leitner O., Moll R., Franke W. W., Geiger B. Monoclonal antibodies to various acidic (type I) cytokeratins of stratified epithelia. Selective markers for stratification and squamous cell carcinomas. Differentiation. 1986;31(2):141–153. doi: 10.1111/j.1432-0436.1986.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Höfler H., Walter G. F., Denk H. Immunohistochemistry of folliculo-stellate cells in normal human adenohypophyses and in pituitary adenomas. Acta Neuropathol. 1984;65(1):35–40. doi: 10.1007/BF00689825. [DOI] [PubMed] [Google Scholar]
- Jao W., Recant W., Swerdlow M. A. Comparative ultrastructure of tubular carcinoma and sclerosing adenosis of the breast. Cancer. 1976 Jul;38(1):180–186. doi: 10.1002/1097-0142(197607)38:1<180::aid-cncr2820380128>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Nagle R. B., Kaufmann M., Maurer C., Böcker W. J. Differential diagnosis of benign epithelial proliferations and carcinomas of the breast using antibodies to cytokeratins. Hum Pathol. 1988 Mar;19(3):276–289. doi: 10.1016/s0046-8177(88)80520-3. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Franke W. W., Moser J. G., Stoffels U. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J. 1983;2(5):735–742. doi: 10.1002/j.1460-2075.1983.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J., Schwechheimer K., Moll R., Franke W. W. Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J Cell Biol. 1984 Mar;98(3):1072–1081. doi: 10.1083/jcb.98.3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M., Stosiek P., van Muijen G. N., Moll R. Cell type heterogeneity of intermediate filament expression in epithelia of the human pituitary gland. Histochemistry. 1989;93(1):93–103. doi: 10.1007/BF00266853. [DOI] [PubMed] [Google Scholar]
- Krepler R., Denk H., Weirich E., Schmid E., Franke W. W. Keratin-like proteins in normal and neoplastic cells of human and rat mammary gland as revealed by immunofluorescence microscopy. Differentiation. 1981;20(3):242–252. doi: 10.1111/j.1432-0436.1981.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Leader M., Collins M., Patel J., Henry K. Vimentin: an evaluation of its role as a tumour marker. Histopathology. 1987 Jan;11(1):63–72. doi: 10.1111/j.1365-2559.1987.tb02609.x. [DOI] [PubMed] [Google Scholar]
- Leoncini P., Cintorino M., Vindigni C., Leoncini L., Armellini D., Bugnoli M., Skalli O., Gabbiani G. Distribution of cytoskeletal and contractile proteins in normal and tumour bearing salivary and lacrimal glands. Virchows Arch A Pathol Anat Histopathol. 1988;412(4):329–337. doi: 10.1007/BF00750259. [DOI] [PubMed] [Google Scholar]
- Liao S. Y., Choi B. H. Expression of glial fibrillary acidic protein by neoplastic cells of müllerian origin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(2):185–193. doi: 10.1007/BF02889962. [DOI] [PubMed] [Google Scholar]
- Lowenthal A., Flament-Durand J., Karcher D., Noppe M., Brion J. P. Glial cells identified by anti-alpha-albumin (anti-GFA) in human pineal gland. J Neurochem. 1982 Mar;38(3):863–865. doi: 10.1111/j.1471-4159.1982.tb08714.x. [DOI] [PubMed] [Google Scholar]
- Memoli V. A., Brown E. F., Gould V. E. Glial fibrillary acidic protein (GFAP) immunoreactivity in peripheral nerve sheath tumors. Ultrastruct Pathol. 1984;7(4):269–275. doi: 10.3109/01913128409141487. [DOI] [PubMed] [Google Scholar]
- Moll R., Achtstätter T., Becht E., Balcarova-Ständer J., Ittensohn M., Franke W. W. Cytokeratins in normal and malignant transitional epithelium. Maintenance of expression of urothelial differentiation features in transitional cell carcinomas and bladder carcinoma cell culture lines. Am J Pathol. 1988 Jul;132(1):123–144. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Cowin P., Kapprell H. P., Franke W. W. Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest. 1986 Jan;54(1):4–25. [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R., Lee I., Gould V. E., Berndt R., Roessner A., Franke W. W. Immunocytochemical analysis of Ewing's tumors. Patterns of expression of intermediate filaments and desmosomal proteins indicate cell type heterogeneity and pluripotential differentiation. Am J Pathol. 1987 May;127(2):288–304. [PMC free article] [PubMed] [Google Scholar]
- Møller M., Ingild A., Bock E. Immunohistochemical demonstration of S-100 protein and GFA protein in interstitial cells of rat pineal gland. Brain Res. 1978 Jan 20;140(1):1–13. doi: 10.1016/0006-8993(78)90234-2. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Böcker W., Davis J. R., Heid H. W., Kaufmann M., Lucas D. O., Jarasch E. D. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986 Jul;34(7):869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Ishida Y., Takahashi K., Suzuki K. Immunohistochemical distribution of S-100 protein and glial fibrillary acidic protein in normal and neoplastic salivary glands. Virchows Arch A Pathol Anat Histopathol. 1985;405(3):299–310. doi: 10.1007/BF00710066. [DOI] [PubMed] [Google Scholar]
- Nakazato Y., Ishizeki J., Takahashi K., Yamaguchi H., Kamei T., Mori T. Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenoma of the salivary glands. Lab Invest. 1982 Jun;46(6):621–626. [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Papasozomenos S. C. Glial fibrillary acidic (GFA) protein-containing cells in the human pineal gland. J Neuropathol Exp Neurol. 1983 Jul;42(4):391–408. doi: 10.1097/00005072-198307000-00003. [DOI] [PubMed] [Google Scholar]
- Pellegrino M. B., Asch B. B., Connolly J. L., Asch H. L. Differential expression of keratins 13 and 16 in normal epithelium, benign lesions, and ductal carcinomas of the human breast determined by the monoclonal antibody Ks8.12. Cancer Res. 1988 Oct 15;48(20):5831–5836. [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. Heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur J Biochem. 1983 May 16;132(3):477–484. doi: 10.1111/j.1432-1033.1983.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Haag D., Jap P., Vooijs P. G. Immunochemical demonstration of keratin and vimentin in cytologic aspirates. Acta Cytol. 1984 Jul-Aug;28(4):385–392. [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S. Co-expression of cytokeratin and vimentin intermediate filament proteins in benign and neoplastic breast epithelium. J Pathol. 1989 Apr;157(4):299–306. doi: 10.1002/path.1711570406. [DOI] [PubMed] [Google Scholar]
- Raymond W. A., Leong A. S. Vimentin--a new prognostic parameter in breast carcinoma? J Pathol. 1989 Jun;158(2):107–114. doi: 10.1002/path.1711580205. [DOI] [PubMed] [Google Scholar]
- Roessmann U., Velasco M. E., Sindely S. D., Gambetti P. Glial fibrillary acidic protein (GFAP) in ependymal cells during development. An immunocytochemical study. Brain Res. 1980 Oct 27;200(1):13–21. doi: 10.1016/0006-8993(80)91090-2. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Brucher J. M. Focal ependymal differentiation in choroid plexus papillomas. An immunoperoxidase study. Acta Neuropathol. 1981;53(1):29–33. doi: 10.1007/BF00697181. [DOI] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 1981 Aug;90(2):435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J., Franke W. W., Schachner M. Immunocytochemical demonstration of vimentin in astrocytes and ependymal cells of developing and adult mouse nervous system. J Cell Biol. 1981 Aug;90(2):435–447. doi: 10.1083/jcb.90.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G., Osborn M., Weber K. Occurrence of two different intermediate filament proteins in the same filament in situ within a human glioma cell line. An immunoelectron microscopical study. Exp Cell Res. 1982 Oct;141(2):385–395. doi: 10.1016/0014-4827(82)90227-0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Osborn M., Weber K. An immunofluorescence microscopical study of the neurofilament triplet proteins, vimentin and glial fibrillary acidic protein within the adult rat brain. Eur J Cell Biol. 1981 Dec;26(1):68–82. [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommers C. L., Walker-Jones D., Heckford S. E., Worland P., Valverius E., Clark R., McCormick F., Stampfer M., Abularach S., Gelmann E. P. Vimentin rather than keratin expression in some hormone-independent breast cancer cell lines and in oncogene-transformed mammary epithelial cells. Cancer Res. 1989 Aug 1;49(15):4258–4263. [PubMed] [Google Scholar]
- Sorenson S. C., Asch B. B., Connolly J. L., Burstein N. A., Asch H. L. Structural distinctions among human breast epithelial cells revealed by the monclonal antikeratin antibodies AE1 and AE3. J Pathol. 1987 Oct;153(2):151–162. doi: 10.1002/path.1711530208. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Qizilbash A. H., Kontozoglou T., Daya A. D., Riddell R. H. An immunohistochemical study of pleomorphic adenomas of the salivary gland: glial fibrillary acidic protein-like immunoreactivity identifies a major myoepithelial component. Hum Pathol. 1988 Jan;19(1):32–40. doi: 10.1016/s0046-8177(88)80313-7. [DOI] [PubMed] [Google Scholar]
- Suess U., Pliska V. Identification of the pituicytes as astroglial cells by indirect immunofluorescence-staining for the glial fibrillary acidic protein. Brain Res. 1981 Sep 21;221(1):27–33. doi: 10.1016/0006-8993(81)91061-1. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Bennett G. S., Toyama Y., Kleinbart F., Holtzer H. Intermediate filament proteins in the developing chick spinal cord. Dev Biol. 1981 Aug;86(1):40–54. doi: 10.1016/0012-1606(81)90313-4. [DOI] [PubMed] [Google Scholar]
- Tascos N. A., Parr J., Gonatas N. K. Immunocytochemical study of the glial fibrillary acidic protein in human neoplasms of the central nervous system. Hum Pathol. 1982 May;13(5):454–458. doi: 10.1016/s0046-8177(82)80028-2. [DOI] [PubMed] [Google Scholar]
- Troyanovsky S. M., Guelstein V. I., Tchipysheva T. A., Krutovskikh V. A., Bannikov G. A. Patterns of expression of keratin 17 in human epithelia: dependency on cell position. J Cell Sci. 1989 Jul;93(Pt 3):419–426. doi: 10.1242/jcs.93.3.419. [DOI] [PubMed] [Google Scholar]
- Velasco M. E., Roessmann U., Gambetti P. The presence of glial fibrillary acidic protein in the human pituitary gland. J Neuropathol Exp Neurol. 1982 Mar;41(2):150–163. doi: 10.1097/00005072-198203000-00005. [DOI] [PubMed] [Google Scholar]
- Viale G., Doglioni C., Dell'Orto P., Zanetti G., Iuzzolino P., Bontempini L., Coggi G. Glial fibrillary acidic protein immunoreactivity in human respiratory tract cartilages and pulmonary chondromatous hamartomas. Am J Pathol. 1988 Nov;133(2):363–373. [PMC free article] [PubMed] [Google Scholar]
- Wang E., Cairncross J. G., Liem R. K. Identification of glial filament protein and vimentin in the same intermediate filament system in human glioma cells. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2102–2106. doi: 10.1073/pnas.81.7.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Armond S. J., Eng L. F., Rubinstein L. J. The application of glial fibrillary acidic (GFA) protein immunohistochemistry in neurooncology. A progress report. Pathol Res Pract. 1980;168(4):374–394. doi: 10.1016/s0344-0338(80)80273-1. [DOI] [PubMed] [Google Scholar]