Abstract
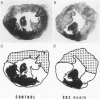
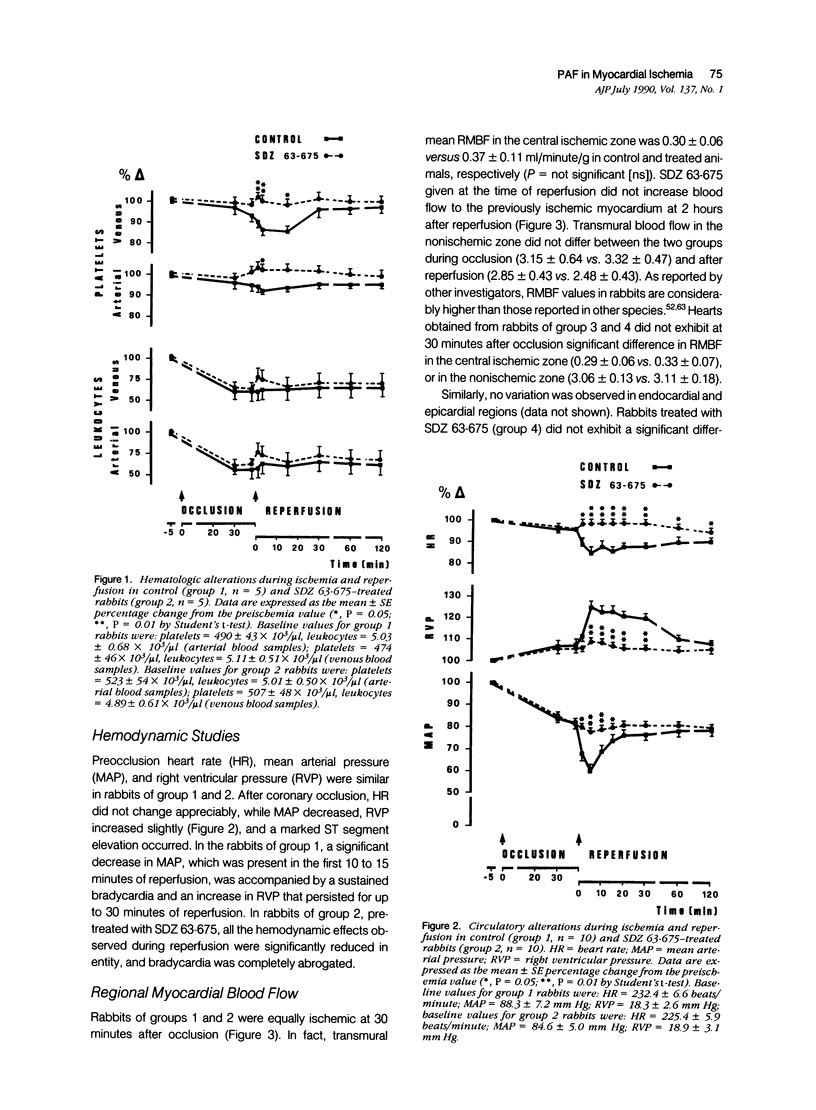
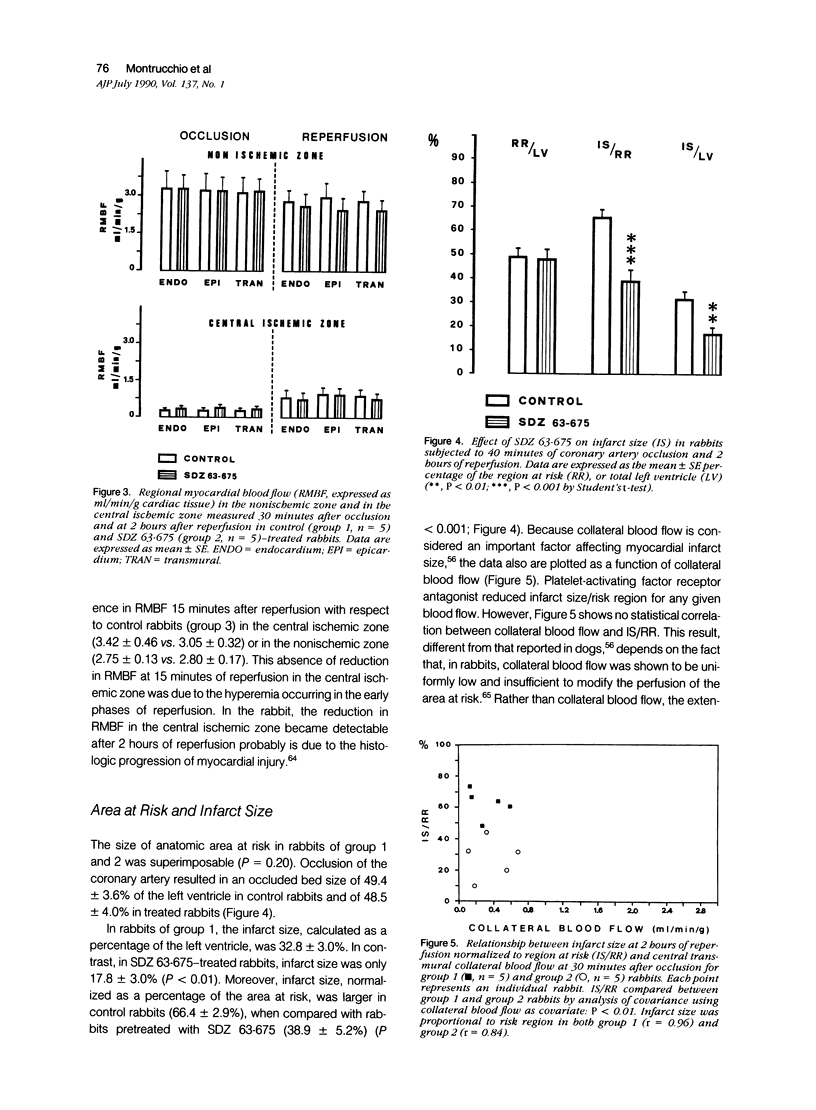
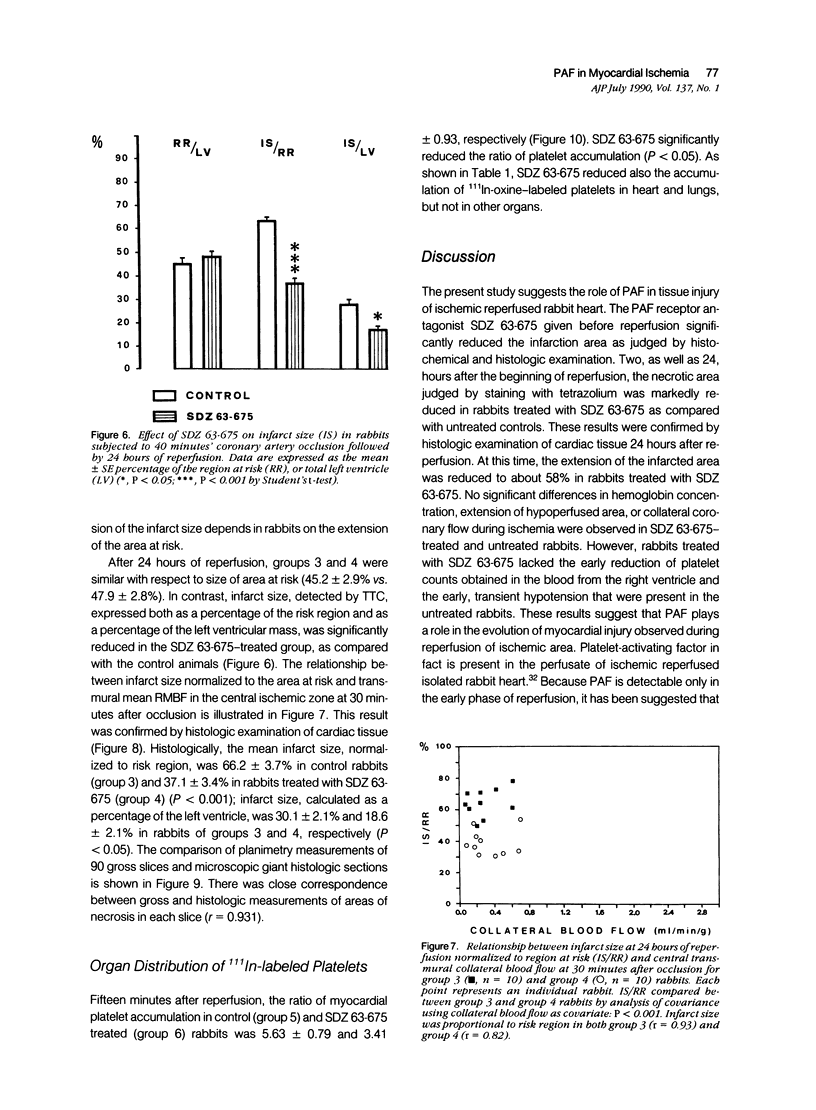
This study shows that the administration of the PAF receptor antagonist SDZ 63.675 (5 mg/kg body weight) before reperfusion significantly reduced the hematologic and hemodynamic alterations, as well as the size of necrotic area in rabbits subjected to 40 minutes of coronary occlusion and reperfusion. Pretreatment with SDZ 63.675 prevented the reduction of platelet counts in the blood obtained from the right ventricle (86.6 +/- 2.8% of the control preischemia value) and the transient bradycardia (85.0 +/- 2.8%), the systemic hypotension (58.0 +/- 2.8%), and the increase in right ventricular pressure (125.0 +/- 3.6%) that were evident in the first minutes of reperfusion in untreated control rabbits. Two as well as 24 hours after reperfusion, the infarct size, judged by staining with tetrazolium, was significantly reduced in rabbits treated with SDZ 63.675 (infarct size in control animals, 66.0 +/- 2.9% and 63.46 +/- 2.09% of the risk region at 2 or 24 hours, respectively, compared with 38.9 +/- 5.2% and 37.11 +/- 2.44% of the risk region at 2 and 24 hours in rabbits treated with SDZ 63.675). This result was confirmed by histologic examination of cardiac tissue 24 hours after reperfusion. In addition, SDZ 63.675 markedly reduced the accumulation of 111In-oxine-labeled platelets that occurs 15 minutes after reperfusion in the central ischemic area of the heart and in the lungs. These results suggest that PAF plays a role in the evolution of myocardial injury observed during reperfusion.
Full text
PDF




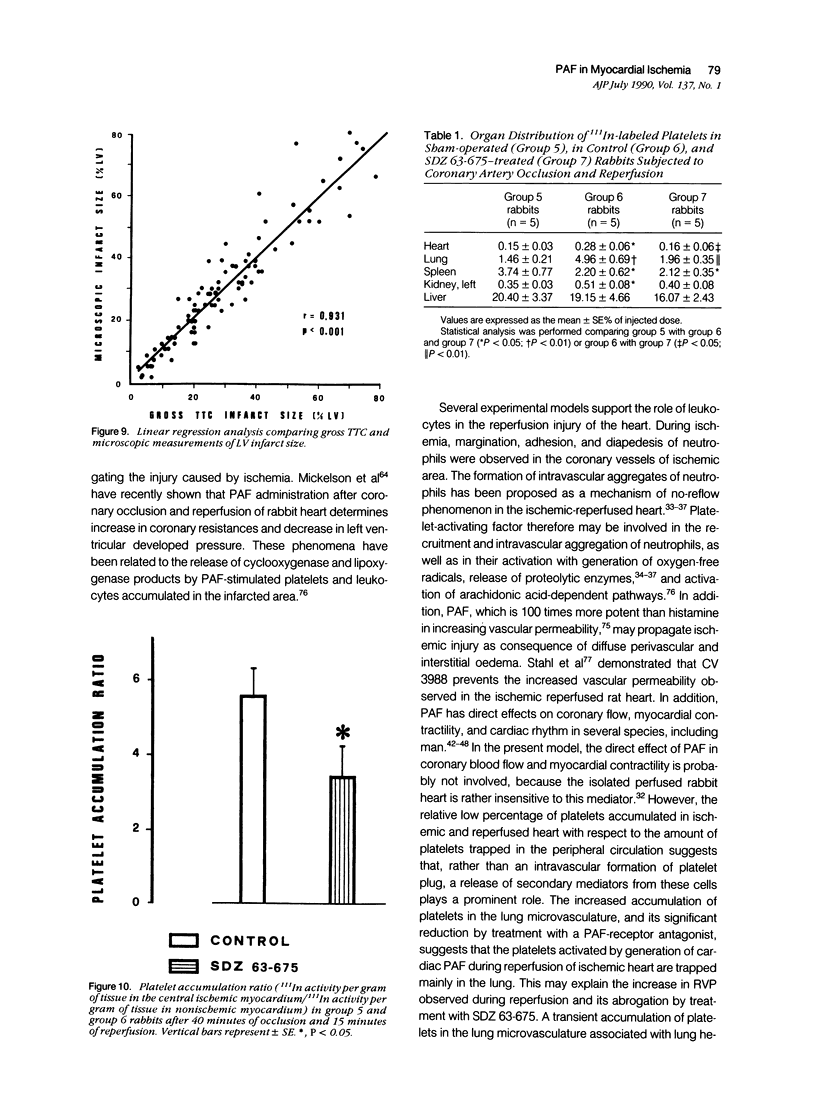




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloatti G., Montrucchio G., Mariano F., Tetta C., De Paulis R., Morea M., Emanuelli G., Camussi G. Effect of platelet-activating factor (PAF) on human cardiac muscle. Int Arch Allergy Appl Immunol. 1986;79(1):108–112. doi: 10.1159/000233953. [DOI] [PubMed] [Google Scholar]
- Alloatti G., Montrucchio G., Mariano F., Tetta C., Emanuelli G., Camussi G. Protective effect of verapamil on the cardiac and circulatory alterations induced by platelet-activating factor. J Cardiovasc Pharmacol. 1987 Feb;9(2):181–186. doi: 10.1097/00005344-198702000-00009. [DOI] [PubMed] [Google Scholar]
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Boullet C., Brink C., Labat C. The actions of Paf-acether (platelet-activating factor) on guinea-pig isolated heart preparations. Br J Pharmacol. 1983 Sep;80(1):81–83. doi: 10.1111/j.1476-5381.1983.tb11052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R., Zhu W. X., Hartley C. J., Michael L. H., Repine J. E., Hess M. L., Kukreja R. C., Roberts R. Attenuation of dysfunction in the postischemic 'stunned' myocardium by dimethylthiourea. Circulation. 1987 Aug;76(2):458–468. doi: 10.1161/01.cir.76.2.458. [DOI] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985 Nov;76(5):1713–1719. doi: 10.1172/JCI112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunwald E., Kloner R. A. The stunned myocardium: prolonged, postischemic ventricular dysfunction. Circulation. 1982 Dec;66(6):1146–1149. doi: 10.1161/01.cir.66.6.1146. [DOI] [PubMed] [Google Scholar]
- Bulkely B. H., Hutchins G. M. Myocardial consequences of coronary artery bypass graft surgery. The paradox of necrosis in areas of revascularization. Circulation. 1977 Dec;56(6):906–913. doi: 10.1161/01.cir.56.6.906. [DOI] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Coda R., Bussolino F., Piacibello W., Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981 Feb;42(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Malavasi F., Tetta C., Piacibello W., Sanavio F., Bussolino F. The release of platelet-activating factor from human endothelial cells in culture. J Immunol. 1983 Nov;131(5):2397–2403. [PubMed] [Google Scholar]
- Camussi G., Alloatti G., Montrucchio G., Meda M., Emanuelli G. Effect of platelet activating factor on guinea-pig papillary muscle. Experientia. 1984 Jul 15;40(7):697–699. doi: 10.1007/BF01949729. [DOI] [PubMed] [Google Scholar]
- Camussi G., Bussolino F., Tetta C., Piacibello W., Aglietta M. Biosynthesis and release of platelet-activating factor from human monocytes. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):245–251. doi: 10.1159/000233331. [DOI] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Bussolino F., Caligaris Cappio F., Coda R., Masera C., Segoloni G. Mediators of immune-complex-induced aggregation of polymorphonuclear neutrophils. II. Platelet-activating factor as the effector substance of immune-induced aggregation. Int Arch Allergy Appl Immunol. 1981;64(1):25–41. doi: 10.1159/000232671. [DOI] [PubMed] [Google Scholar]
- Carlson R. E., Schott R. J., Buda A. J. Neutrophil depletion fails to modify myocardial no reflow and functional recovery after coronary reperfusion. J Am Coll Cardiol. 1989 Dec;14(7):1803–1813. doi: 10.1016/0735-1097(89)90036-3. [DOI] [PubMed] [Google Scholar]
- Chatelain P., Latour J. G., Tran D., de Lorgeril M., Dupras G., Bourassa M. Neutrophil accumulation in experimental myocardial infarcts: relation with extent of injury and effect of reperfusion. Circulation. 1987 May;75(5):1083–1090. doi: 10.1161/01.cir.75.5.1083. [DOI] [PubMed] [Google Scholar]
- Chignard M., Le Couedic J. P., Tence M., Vargaftig B. B., Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979 Jun 28;279(5716):799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Walsh C. E., Thomas M. J., Wykle R. L., DeChatelet L. R., Waite B. M. Platelet activating factor. Stimulation of the lipoxygenase pathway in polymorphonuclear leukocytes by 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine. J Biol Chem. 1982 May 25;257(10):5402–5407. [PubMed] [Google Scholar]
- Coker S. J. Further evidence that thromboxane exacerbates arrhythmias: effects of UK38485 during coronary artery occlusion and reperfusion in anaesthetized greyhounds. J Mol Cell Cardiol. 1984 Jul;16(7):633–641. doi: 10.1016/s0022-2828(84)80627-6. [DOI] [PubMed] [Google Scholar]
- Coker S. J., Parratt J. R. Effects of dazoxiben on arrhythmias and ventricular fibrillation induced by coronary artery occlusion and reperfusion in anaesthetised greyhounds. Br J Clin Pharmacol. 1983;15 (Suppl 1):87S–95S. doi: 10.1111/j.1365-2125.1983.tb02115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D. K., Engelman R. M., Rousou J. A., Breyer R. H., Otani H., Lemeshow S. Role of membrane phospholipids in myocardial injury induced by ischemia and reperfusion. Am J Physiol. 1986 Jul;251(1 Pt 2):H71–H79. doi: 10.1152/ajpheart.1986.251.1.H71. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Engler R. Consequences of activation and adenosine-mediated inhibition of granulocytes during myocardial ischemia. Fed Proc. 1987 May 15;46(7):2407–2412. [PubMed] [Google Scholar]
- Engler R., Gilpin E. Can superoxide dismutase alter myocardial infarct size? Circulation. 1989 May;79(5):1137–1142. doi: 10.1161/01.cir.79.5.1137. [DOI] [PubMed] [Google Scholar]
- Ferrari R., Ceconi C., Curello S., Guarnieri C., Caldarera C. M., Albertini A., Visioli O. Oxygen-mediated myocardial damage during ischaemia and reperfusion: role of the cellular defences against oxygen toxicity. J Mol Cell Cardiol. 1985 Oct;17(10):937–945. doi: 10.1016/s0022-2828(85)80074-2. [DOI] [PubMed] [Google Scholar]
- Feuerstein G., Boyd L. M., Ezra D., Goldstein R. E. Effect of platelet-activating factor on coronary circulation of the domestic pig. Am J Physiol. 1984 Mar;246(3 Pt 2):H466–H471. doi: 10.1152/ajpheart.1984.246.3.H466. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Bingham S., Kopelman H. A., Wehr C., Sandler M. P., Kolodgie F., Vaughn W. K., Friesinger G. C., Virmani R. Reduction of infarct size with intracoronary perfluorochemical in a canine preparation of reperfusion. Circulation. 1985 May;71(5):1060–1068. doi: 10.1161/01.cir.71.5.1060. [DOI] [PubMed] [Google Scholar]
- Forman M. B., Puett D. W., Cates C. U., McCroskey D. E., Beckman J. K., Greene H. L., Virmani R. Glutathione redox pathway and reperfusion injury. Effect of N-acetylcysteine on infarct size and ventricular function. Circulation. 1988 Jul;78(1):202–213. doi: 10.1161/01.cir.78.1.202. [DOI] [PubMed] [Google Scholar]
- Frame L. H., Lopez J. A., Khaw B. A., Fallon J. T., Haber E., Powell W. J., Jr Early membrane damage during coronary reperfusion in dogs. Detection by radiolabeled anticardiac myosin (Fab')2. J Clin Invest. 1983 Aug;72(2):535–544. doi: 10.1172/JCI111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginks W. R., Sybers H. D., Maroko P. R., Covell J. W., Sobel B. E., Ross J., Jr Coronary artery reperfusion. II. Reduction of myocardial infarct size at 1 week after the coronary occlusion. J Clin Invest. 1972 Oct;51(10):2717–2723. doi: 10.1172/JCI107091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golino P., Maroko P. R., Carew T. E. Efficacy of platelet depletion in counteracting the detrimental effect of acute hypercholesterolemia on infarct size and the no-reflow phenomenon in rabbits undergoing coronary artery occlusion-reperfusion. Circulation. 1987 Jul;76(1):173–180. doi: 10.1161/01.cir.76.1.173. [DOI] [PubMed] [Google Scholar]
- Halonen M., Palmer J. D., Lohman I. C., McManus L. M., Pinckard R. N. Respiratory and circulatory alterations induced by acetyl glyceryl ether phosphorylcholine, a mediator of IgE anaphylaxis in the rabbit. Am Rev Respir Dis. 1980 Dec;122(6):915–924. doi: 10.1164/arrd.1980.122.6.915. [DOI] [PubMed] [Google Scholar]
- Handley D. A., Van Valen R. G., Winslow C. M., Tomesch J. C., Saunders R. N. In vitro and in vivo pharmacological profiles of the PAF receptor antagonist SRI 63-675. Thromb Haemost. 1987 Apr 7;57(2):187–190. [PubMed] [Google Scholar]
- Hess M. L., Manson N. H. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984 Nov;16(11):969–985. doi: 10.1016/s0022-2828(84)80011-5. [DOI] [PubMed] [Google Scholar]
- Heymann M. A., Payne B. D., Hoffman J. I., Rudolph A. M. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977 Jul-Aug;20(1):55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Hill-Zobel R. L., Scheffel U., McIntyre P. A., Tsan M. F. 111In oxine-labeled rabbit platelets: in vivo distribution and sites of destruction. Blood. 1983 Jan;61(1):149–153. [PubMed] [Google Scholar]
- Hoffman D. R., Hajdu J., Snyder F. Cytotoxicity of platelet activating factor and related alkyl-phospholipid analogs in human leukemia cells, polymorphonuclear neutrophils, and skin fibroblasts. Blood. 1984 Mar;63(3):545–552. [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Satouchi K., Hanahan D. J., Pinckard R. N. Vasoactive properties of acetyl glyceryl ether phosphorylcholine and analogues. Lab Invest. 1982 Apr;46(4):422–427. [PubMed] [Google Scholar]
- Jackson C. V., Schumacher W. A., Kunkel S. L., Driscoll E. M., Lucchesi B. R. Platelet-activating factor and the release of a platelet-derived coronary artery vasodilator substance in the canine. Circ Res. 1986 Feb;58(2):218–229. doi: 10.1161/01.res.58.2.218. [DOI] [PubMed] [Google Scholar]
- Jolly S. R., Kane W. J., Bailie M. B., Abrams G. D., Lucchesi B. R. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984 Mar;54(3):277–285. doi: 10.1161/01.res.54.3.277. [DOI] [PubMed] [Google Scholar]
- KLIONSKY B. Myocardial ischemia and early infarction: a histochemical study. Am J Pathol. 1960 May;36:575–591. [PMC free article] [PubMed] [Google Scholar]
- Karsch K. R., Hofmann M., Rentrop K. P., Blanke H., Schaper W. Thrombolysis in acute experimental myocardial infarction. J Am Coll Cardiol. 1983 Feb;1(2 Pt 1):427–435. doi: 10.1016/s0735-1097(83)80070-9. [DOI] [PubMed] [Google Scholar]
- Khaja F., Walton J. A., Jr, Brymer J. F., Lo E., Osterberger L., O'Neill W. W., Colfer H. T., Weiss R., Lee T., Kurian T. Intracoronary fibrinolytic therapy in acute myocardial infarction. Report of a prospective randomized trial. N Engl J Med. 1983 Jun 2;308(22):1305–1311. doi: 10.1056/NEJM198306023082201. [DOI] [PubMed] [Google Scholar]
- Kloner R. A. Do neutrophils mediate the phenomenon of stunned myocardium? J Am Coll Cardiol. 1989 Apr;13(5):1164–1166. doi: 10.1016/0735-1097(89)90279-9. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ellis S. G., Lange R., Braunwald E. Studies of experimental coronary artery reperfusion. Effects on infarct size, myocardial function, biochemistry, ultrastructure and microvascular damage. Circulation. 1983 Aug;68(2 Pt 2):I8–15. [PubMed] [Google Scholar]
- Kloner R. A. No reflow revisited. J Am Coll Cardiol. 1989 Dec;14(7):1814–1815. doi: 10.1016/0735-1097(89)90037-5. [DOI] [PubMed] [Google Scholar]
- Koren G., Weiss A. T., Hasin Y., Appelbaum D., Welber S., Rozenman Y., Lotan C., Mosseri M., Sapoznikov D., Luria M. H. Prevention of myocardial damage in acute myocardial ischemia by early treatment with intravenous streptokinase. N Engl J Med. 1985 Nov 28;313(22):1384–1389. doi: 10.1056/NEJM198511283132204. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Mentley R., Sun J. Z. Potentiation of myocardial salvage by tissue type plasminogen activator in combination with a thromboxane synthetase inhibitor in ischemic cat myocardium. Circ Res. 1988 Sep;63(3):621–627. doi: 10.1161/01.res.63.3.621. [DOI] [PubMed] [Google Scholar]
- Leprán I., Lefer A. M. Ischemia aggravating effects of platelet-activating factor in acute myocardial ischemia. Basic Res Cardiol. 1985 Mar-Apr;80(2):135–141. doi: 10.1007/BF01910460. [DOI] [PubMed] [Google Scholar]
- Levi R., Burke J. A., Guo Z. G., Hattori Y., Hoppens C. M., McManus L. M., Hanahan D. J., Pinckard R. N. Acetyl glyceryl ether phosphorylcholine (AGEPC). A putative mediator of cardiac anaphylaxis in the guinea pig. Circ Res. 1984 Feb;54(2):117–124. doi: 10.1161/01.res.54.2.117. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Whatley R. E., Cain P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest. 1988 Dec;82(6):2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotner G. Z., Lynch J. M., Betz S. J., Henson P. M. Human neutrophil-derived platelet activating factor. J Immunol. 1980 Feb;124(2):676–684. [PubMed] [Google Scholar]
- Lundberg C., Marceau F., Hugli T. E. C5a-induced hemodynamic and hematologic changes in the rabbit. Role of cyclooxygenase products and polymorphonuclear leukocytes. Am J Pathol. 1987 Sep;128(3):471–483. [PMC free article] [PubMed] [Google Scholar]
- McManus L. M., Pinckard R. N. Kinetics of acetyl glyceryl ether phosphorylcholine (AGEPC)-induced acute lung alterations in the rabbit. Am J Pathol. 1985 Oct;121(1):55–68. [PMC free article] [PubMed] [Google Scholar]
- Mickelson J. K., Simpson P. J., Lucchesi B. R. Myocardial dysfunction and coronary vasoconstriction induced by platelet-activating factor in the post-infarcted rabbit isolated heart. J Mol Cell Cardiol. 1988 Jun;20(6):547–561. doi: 10.1016/s0022-2828(88)80081-6. [DOI] [PubMed] [Google Scholar]
- Montrucchio G., Alloatti G., Tetta C., De Luca R., Saunders R. N., Emanuelli G., Camussi G. Release of platelet-activating factor from ischemic-reperfused rabbit heart. Am J Physiol. 1989 Apr;256(4 Pt 2):H1236–H1246. doi: 10.1152/ajpheart.1989.256.4.H1236. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Fornabaio D. Thromboxane synthetase inhibitors reduce infarct size by a platelet-dependent, aspirin-sensitive mechanism. Circ Res. 1988 Apr;62(4):668–678. doi: 10.1161/01.res.62.4.668. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Salmon J. A., Kraemer R. Leukocyte-derived metabolites of arachidonic acid in ischemia-induced myocardial injury. Fed Proc. 1987 May 15;46(7):2422–2433. [PubMed] [Google Scholar]
- Mullane K., Hatala M. A., Kraemer R., Sessa W., Westlin W. Myocardial salvage induced by REV-5901: an inhibitor and antagonist of the leukotrienes. J Cardiovasc Pharmacol. 1987 Oct;10(4):398–406. doi: 10.1097/00005344-198710000-00004. [DOI] [PubMed] [Google Scholar]
- Neutze J. M., Wyler F., Rudolph A. M. Use of radioactive microspheres to assess distribution of cardiac output in rabbits. Am J Physiol. 1968 Aug;215(2):486–495. doi: 10.1152/ajplegacy.1968.215.2.486. [DOI] [PubMed] [Google Scholar]
- O'Neill W., Timmis G. C., Bourdillon P. D., Lai P., Ganghadarhan V., Walton J., Jr, Ramos R., Laufer N., Gordon S., Schork M. A. A prospective randomized clinical trial of intracoronary streptokinase versus coronary angioplasty for acute myocardial infarction. N Engl J Med. 1986 Mar 27;314(13):812–818. doi: 10.1056/NEJM198603273141303. [DOI] [PubMed] [Google Scholar]
- Oates P. S., Bruce N. W., Morgan R. G. Pancreatic blood flow in the rat during enlargement, involution, and cholecystokinin treatment. Am J Physiol. 1984 Nov;247(5 Pt 1):G457–G462. doi: 10.1152/ajpgi.1984.247.5.G457. [DOI] [PubMed] [Google Scholar]
- Otani H., Engelman R. M., Breyer R. H., Rousou J. A., Lemeshow S., Das D. K. Mepacrine, a phospholipase inhibitor. A potential tool for modifying myocardial reperfusion injury. J Thorac Cardiovasc Surg. 1986 Aug;92(2):247–254. [PubMed] [Google Scholar]
- Otani H., Engelman R. M., Rousou J. A., Breyer R. H., Das D. K. Enhanced prostaglandin synthesis due to phospholipid breakdown in ischemic-reperfused myocardium. Control of its production by a phospholipase inhibitor or free radical scavengers. J Mol Cell Cardiol. 1986 Sep;18(9):953–961. doi: 10.1016/s0022-2828(86)80009-8. [DOI] [PubMed] [Google Scholar]
- Otani H., Prasad M. R., Engelman R. M., Otani H., Cordis G. A., Das D. K. Enhanced phosphodiesteratic breakdown and turnover of phosphoinositides during reperfusion of ischemic rat heart. Circ Res. 1988 Nov;63(5):930–936. doi: 10.1161/01.res.63.5.930. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyklenk K., Kloner R. A. "Reperfusion injury" by oxygen-derived free radicals? Effect of superoxide dismutase plus catalase, given at the time of reperfusion, on myocardial infarct size, contractile function, coronary microvasculature, and regional myocardial blood flow. Circ Res. 1989 Jan;64(1):86–96. doi: 10.1161/01.res.64.1.86. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B., Cobb F. R., Murdock R. H., Greenfield J. C., Jr, Becker L. C., Bulkley B. H., Hutchins G. M., Schwartz R. P., Jr, Bailey K. R. Animal models for protecting ischemic myocardium: results of the NHLBI Cooperative Study. Comparison of unconscious and conscious dog models. Circ Res. 1985 May;56(5):651–665. doi: 10.1161/01.res.56.5.651. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979 Jun;40(6):633–644. [PubMed] [Google Scholar]
- Reimer K. A., Lowe J. E., Rasmussen M. M., Jennings R. B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977 Nov;56(5):786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W. Capillary plugging by granulocytes and the no-reflow phenomenon in the microcirculation. Fed Proc. 1987 May 15;46(7):2397–2401. [PubMed] [Google Scholar]
- Serruys P. W., Simoons M. L., Suryapranata H., Vermeer F., Wijns W., van den Brand M., Bär F., Zwaan C., Krauss X. H., Remme W. J. Preservation of global and regional left ventricular function after early thrombolysis in acute myocardial infarction. J Am Coll Cardiol. 1986 Apr;7(4):729–742. doi: 10.1016/s0735-1097(86)80330-8. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Stahl G. L., Terashita Z., Lefer A. M. Role of platelet activating factor in propagation of cardiac damage during myocardial ischemia. J Pharmacol Exp Ther. 1988 Mar;244(3):898–904. [PubMed] [Google Scholar]
- Tamura Y., Chi L. G., Driscoll E. M., Jr, Hoff P. T., Freeman B. A., Gallagher K. P., Lucchesi B. R. Superoxide dismutase conjugated to polyethylene glycol provides sustained protection against myocardial ischemia/reperfusion injury in canine heart. Circ Res. 1988 Nov;63(5):944–959. doi: 10.1161/01.res.63.5.944. [DOI] [PubMed] [Google Scholar]
- Thakur M. L., Welch M. J., Joist J. H., Coleman R. E. Indium-LLL labeled platelets: studies on preparation and evaluation of in vitro and in vivo functions. Thromb Res. 1976 Oct;9(4):345–357. doi: 10.1016/0049-3848(76)90135-3. [DOI] [PubMed] [Google Scholar]
- Van de Werf F., Ludbrook P. A., Bergmann S. R., Tiefenbrunn A. J., Fox K. A., de Geest H., Verstraete M., Collen D., Sobel B. E. Coronary thrombolysis with tissue-type plasminogen activator in patients with evolving myocardial infarction. N Engl J Med. 1984 Mar 8;310(10):609–613. doi: 10.1056/NEJM198403083101001. [DOI] [PubMed] [Google Scholar]
- Van de Werf F., Vanhaecke J., de Geest H., Verstraete M., Collen D. Coronary thrombolysis with recombinant single-chain urokinase-type plasminogen activator in patients with acute myocardial infarction. Circulation. 1986 Nov;74(5):1066–1070. doi: 10.1161/01.cir.74.5.1066. [DOI] [PubMed] [Google Scholar]
- Warren D. J., Ledingham J. G. Measurement of cardiac output distribution using microspheres. Some practical and theoretical considerations. Cardiovasc Res. 1974 Jul;8(4):570–581. doi: 10.1093/cvr/8.4.570. [DOI] [PubMed] [Google Scholar]
- Yasaka T., Boxer L. A., Baehner R. L. Monocyte aggregation and superoxide anion release in response to formyl-methionyl-leucyl-phenylalanine (FMLP) and platelet-activating factor (PAF). J Immunol. 1982 May;128(5):1939–1944. [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]