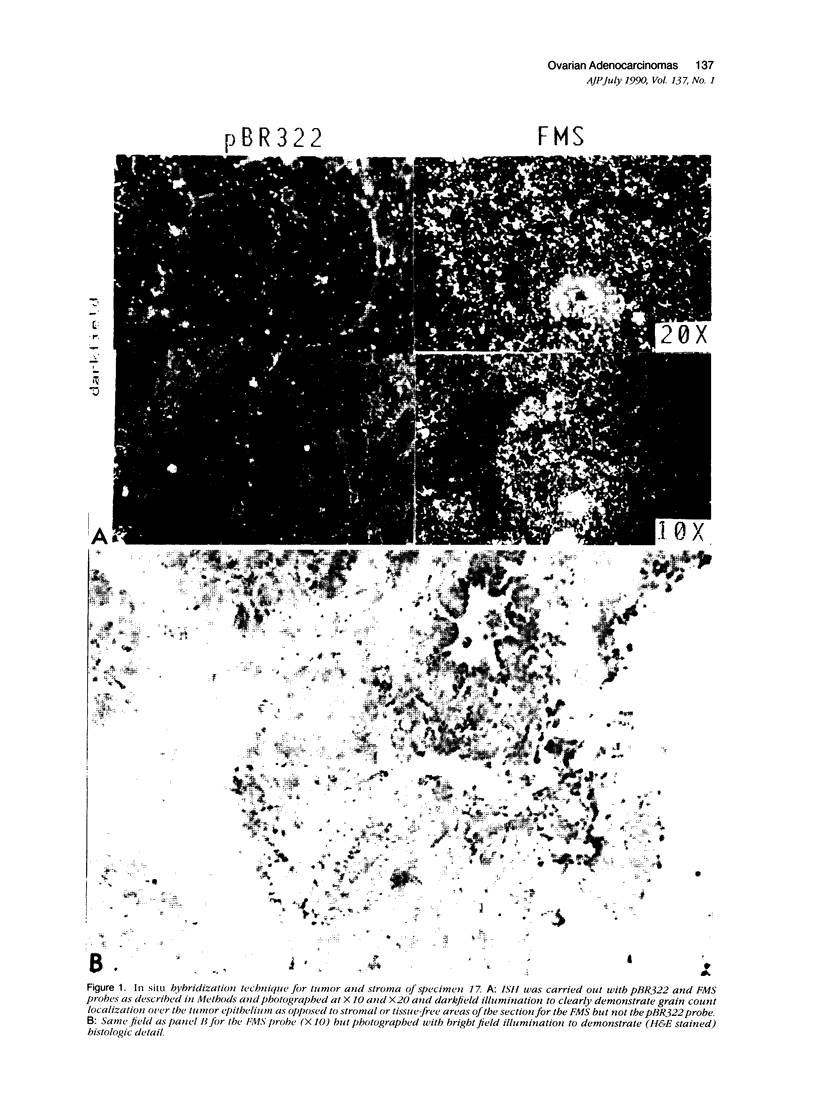
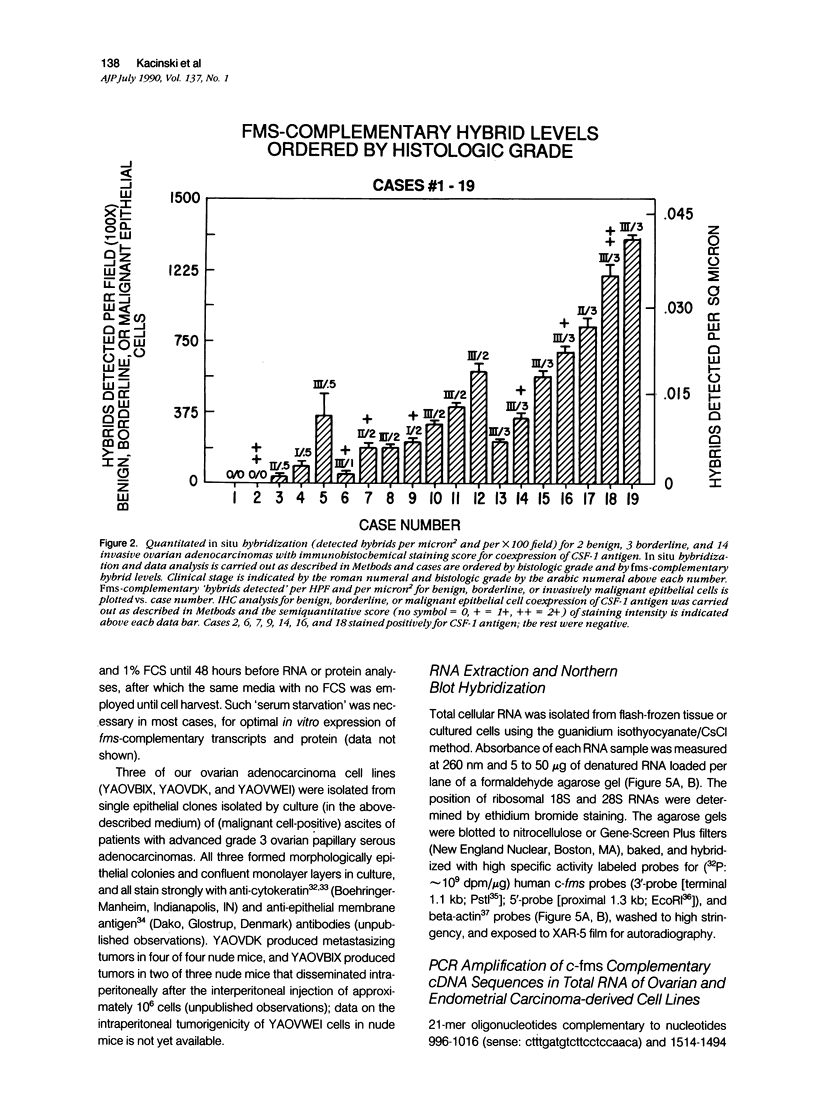
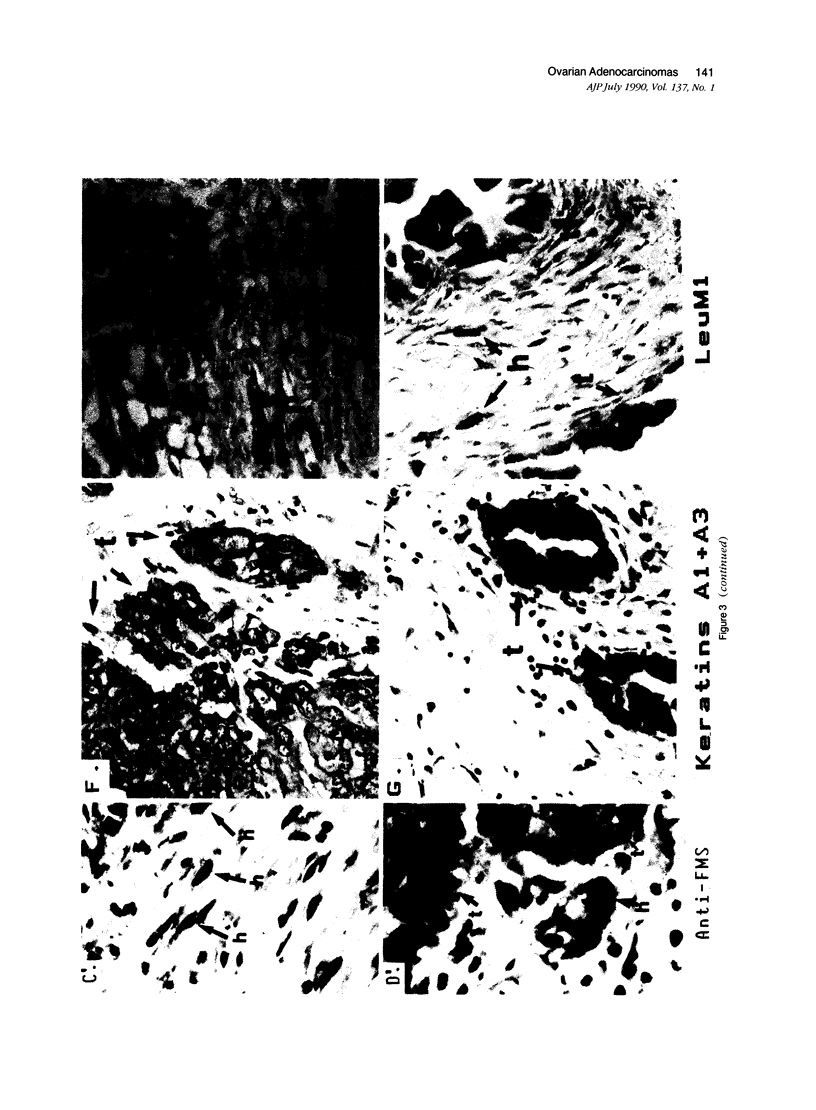
Abstract
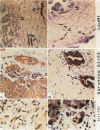
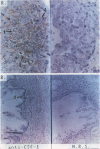
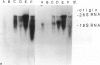
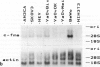
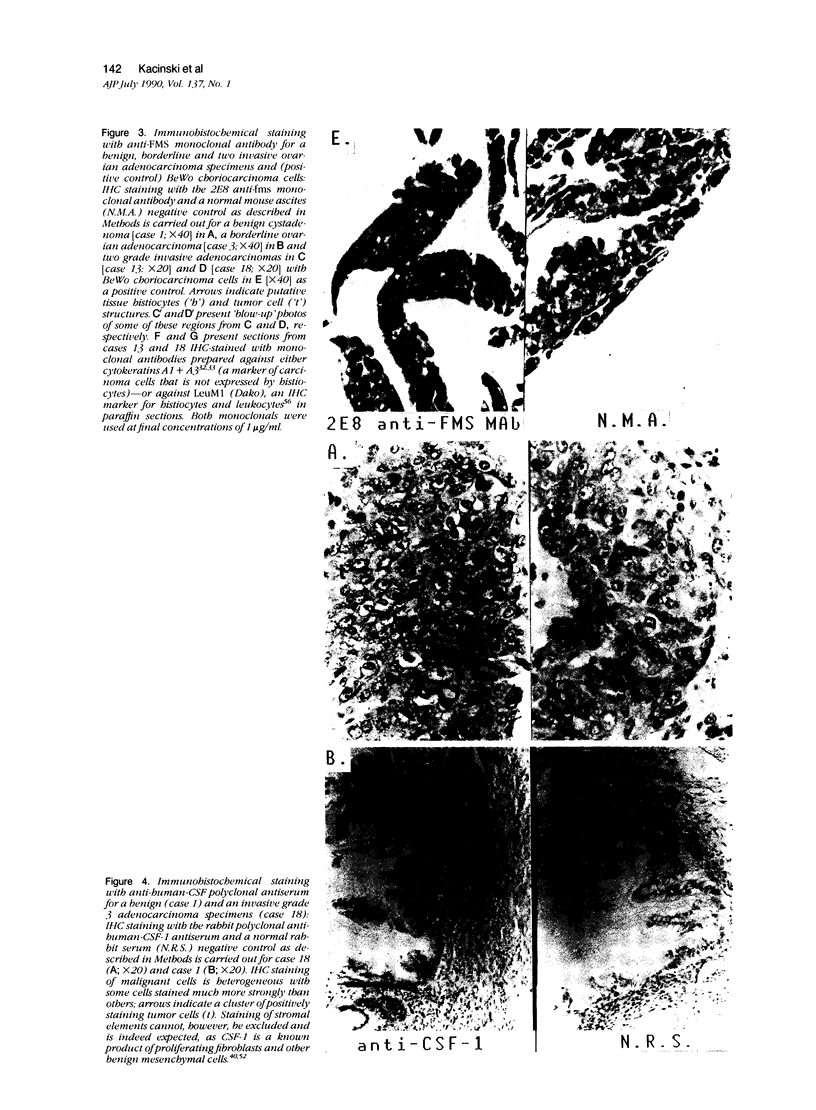
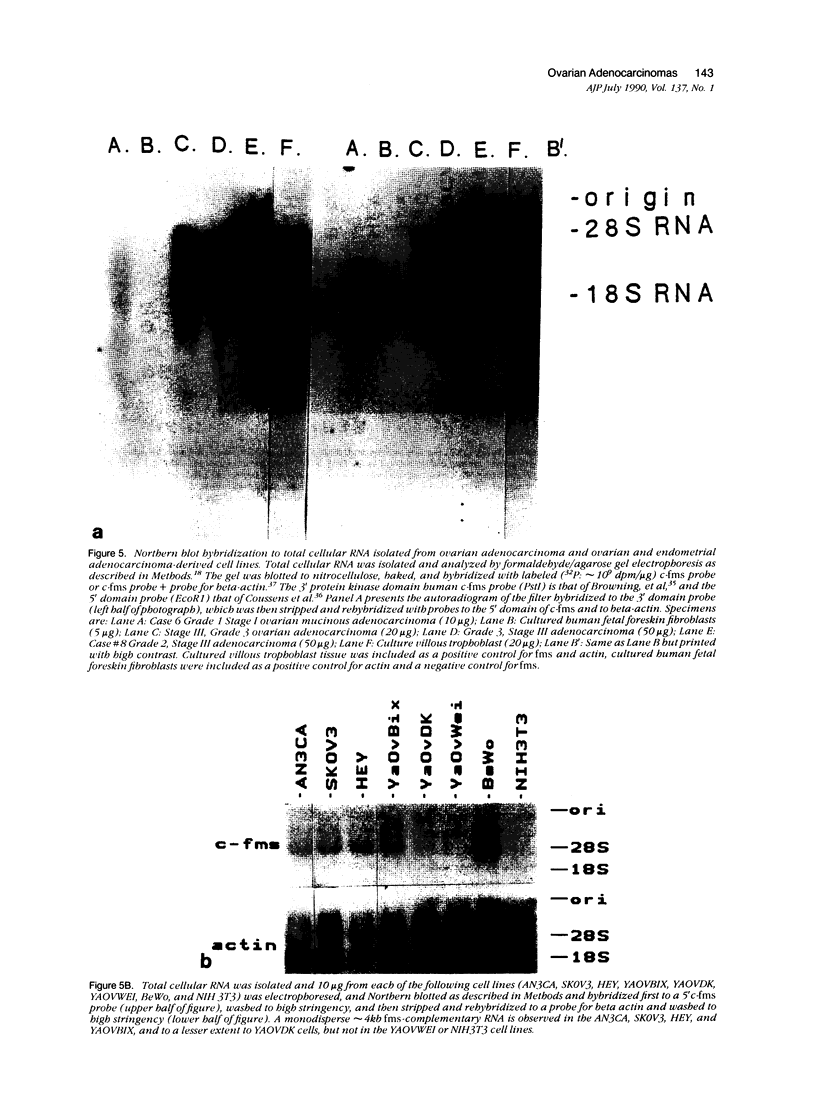
In earlier studies of oncogene expression in ovarian and endometrial neoplasms, the authors reported that high tumor levels of fms-complementary transcripts correlate with high histologic grade and advanced clinical stage presentations. In this communication, they pursue these initial clinicopathologic investigations to demonstrate by in situ hybridization and immunohistochemistry that malignant epithelial cells of 14 of 14 invasive adenocarcinomas of the ovary express fms-complementary transcripts. By Northern blotting and by reverse transcription, followed by polymerase chain reaction amplification, the authors also were able to demonstrate fms transcript expression in several ovarian and endometrial carcinoma-derived cell lines. Because about half (6/14) of the invasive adenocarcinoma specimens were shown to coexpress fms and colony-stimulating factor 1, the authors propose that the expression of this lymphohematopoietic cytokine and its receptor by ovarian adenocarcinomas could contribute totheir proliferative and invasive characteristics in vivo.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartocci A., Pollard J. W., Stanley E. R. Regulation of colony-stimulating factor 1 during pregnancy. J Exp Med. 1986 Sep 1;164(3):956–961. doi: 10.1084/jem.164.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdel W. E., Danhauser-Riedl S., Steinhauser G., Winton E. F. Various human hematopoietic growth factors (interleukin-3, GM-CSF, G-CSF) stimulate clonal growth of nonhematopoietic tumor cells. Blood. 1989 Jan;73(1):80–83. [PubMed] [Google Scholar]
- Browning P. J., Bunn H. F., Cline A., Shuman M., Nienhuis A. W. "Replacement" of COOH-terminal truncation of v-fms with c-fms sequences markedly reduces transformation potential. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7800–7804. doi: 10.1073/pnas.83.20.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buick R. N., Pullano R., Trent J. M. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985 Aug;45(8):3668–3676. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Day T. G., Jr, Gallager H. S., Rutledge F. N. Epithelial carcinoma of the ovary:prognostic importance of histologic grade. Natl Cancer Inst Monogr. 1975 Oct;42:15–21. [PubMed] [Google Scholar]
- Dedhar S., Gaboury L., Galloway P., Eaves C. Human granulocyte-macrophage colony-stimulating factor is a growth factor active on a variety of cell types of nonhemopoietic origin. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9253–9257. doi: 10.1073/pnas.85.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOULDS L. The experimental study of tumor progression: a review. Cancer Res. 1954 Jun;14(5):327–339. [PubMed] [Google Scholar]
- Feldman M., Eisenbach L. What makes a tumor cell metastatic? Sci Am. 1988 Nov;259(5):60-5, 68, 85. doi: 10.1038/scientificamerican1188-60. [DOI] [PubMed] [Google Scholar]
- Godard C. M. Improved method for detection of cellular transcripts by in situ hybridization. Detection of virus-specific RNA in rous sarcoma virus-infected cells by in situ hybridization to cDNA. Histochemistry. 1983;77(1):123–131. doi: 10.1007/BF00496643. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Braverman M., Salafia C., Buckley P. The phenotype of human placental macrophages and its variation with gestational age. Am J Pathol. 1988 Dec;133(3):648–659. [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Horiguchi J., Sherman M. L., Sampson-Johannes A., Weber B. L., Kufe D. W. CSF-1 and C-FMS gene expression in human carcinoma cell lines. Biochem Biophys Res Commun. 1988 Nov 30;157(1):395–401. doi: 10.1016/s0006-291x(88)80060-3. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Kohorn E. I., Mittal K., Bloodgood R. S., Donahue J., Kramer C. A., Fischer D., Edwards R., Chambers S. K. Oncogene expression in vivo by ovarian adenocarcinomas and mixed-mullerian tumors. Yale J Biol Med. 1989 Jul-Aug;62(4):379–392. [PMC free article] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Mittal K., Kohorn E. I., Bloodgood R. S., Donahue J., Donofrio L., Edwards R., Schwartz P. E., Chambers J. T. High level expression of fms proto-oncogene mRNA is observed in clinically aggressive human endometrial adenocarcinomas. Int J Radiat Oncol Biol Phys. 1988 Oct;15(4):823–829. doi: 10.1016/0360-3016(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Stanley E. R., Carter D., Chambers J. T., Chambers S. K., Kohorn E. I., Schwartz P. E. Circulating levels of CSF-1 (M-CSF) a lymphohematopoietic cytokine may be a useful marker of disease status in patients with malignant ovarian neoplasms. Int J Radiat Oncol Biol Phys. 1989 Jul;17(1):159–164. doi: 10.1016/0360-3016(89)90383-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Ladner M. B., Wang A. M., Van Arsdell J., Warren M. K., Coyne M. Y., Schweickart V. L., Lee M. T., Wilson K. J., Boosman A. Molecular cloning of a complementary DNA encoding human macrophage-specific colony-stimulating factor (CSF-1). Science. 1985 Oct 18;230(4723):291–296. doi: 10.1126/science.2996129. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. M., Schwab M., Westaway D., Varmus H. E. Augmented expression of normal c-myc is sufficient for cotransformation of rat embryo cells with a mutant ras gene. Mol Cell Biol. 1985 Dec;5(12):3345–3356. doi: 10.1128/mcb.5.12.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols R. F., Garvin A. J., Costa J., Simon R. M., Young R. C. Advanced ovarian cancer: correlation of histologic grade with response to therapy and survival. Cancer. 1980 Feb;45(3):572–581. doi: 10.1002/1097-0142(19800201)45:3<572::aid-cncr2820450325>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968 Jul;28(7):1231–1236. [PubMed] [Google Scholar]
- Pinkus G. S., Kurtin P. J. Epithelial membrane antigen--a diagnostic discriminant in surgical pathology: immunohistochemical profile in epithelial, mesenchymal, and hematopoietic neoplasms using paraffin sections and monoclonal antibodies. Hum Pathol. 1985 Sep;16(9):929–940. doi: 10.1016/s0046-8177(85)80132-5. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Bartocci A., Arceci R., Orlofsky A., Ladner M. B., Stanley E. R. Apparent role of the macrophage growth factor, CSF-1, in placental development. Nature. 1987 Dec 3;330(6147):484–486. doi: 10.1038/330484a0. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Eng R., Shadduck R. K., Waheed A., Ben-Avram C. M., Shively J. E., Lusis A. J. Cloning and tissue-specific expression of mouse macrophage colony-stimulating factor mRNA. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1157–1161. doi: 10.1073/pnas.84.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan S., Xu F. J., Brandt S. J., Niedel J. E., Bast R. C., Jr, Brown E. L. Constitutive production of macrophage colony-stimulating factor by human ovarian and breast cancer cell lines. J Clin Invest. 1989 Mar;83(3):921–926. doi: 10.1172/JCI113977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Sacca R., Furman W. L., Roussel M. F., Holt J. T., Nienhuis A. W., Stanley E. R., Sherr C. J. Expression of the human c-fms proto-oncogene product (colony-stimulating factor-1 receptor) on peripheral blood mononuclear cells and choriocarcinoma cell lines. J Clin Invest. 1986 Jun;77(6):1740–1746. doi: 10.1172/JCI112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully R. E. Ovarian tumors. A review. Am J Pathol. 1977 Jun;87(3):686–720. [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. Action of the colony-stimulating factor, CSF-1. Ciba Found Symp. 1986;118:29–41. doi: 10.1002/9780470720998.ch3. [DOI] [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Stuart S. G., Simister N. E., Clarkson S. B., Kacinski B. M., Shapiro M., Mellman I. Human IgG Fc receptor (hFcRII; CD32) exists as multiple isoforms in macrophages, lymphocytes and IgG-transporting placental epithelium. EMBO J. 1989 Dec 1;8(12):3657–3666. doi: 10.1002/j.1460-2075.1989.tb08540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C., Nettesheim P., Barrett J. C., Gilmer T. M. Expression of a fms-related oncogene in carcinogen-induced neoplastic epithelial cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1804–1808. doi: 10.1073/pnas.84.7.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Eichner R., Sun T. T. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol. 1984 Apr;98(4):1397–1406. doi: 10.1083/jcb.98.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Escobedo J. A., Kuang W. J., Yang-Feng T. L., Daniel T. O., Tremble P. M., Chen E. Y., Ando M. E., Harkins R. N., Francke U. Structure of the receptor for platelet-derived growth factor helps define a family of closely related growth factor receptors. Nature. 1986 Sep 18;323(6085):226–232. doi: 10.1038/323226a0. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee L. D., Kacinski B. M., Carter D. Oncogene structure, function, and expression in breast cancer. Semin Diagn Pathol. 1989 May;6(2):110–125. [PubMed] [Google Scholar]