Abstract
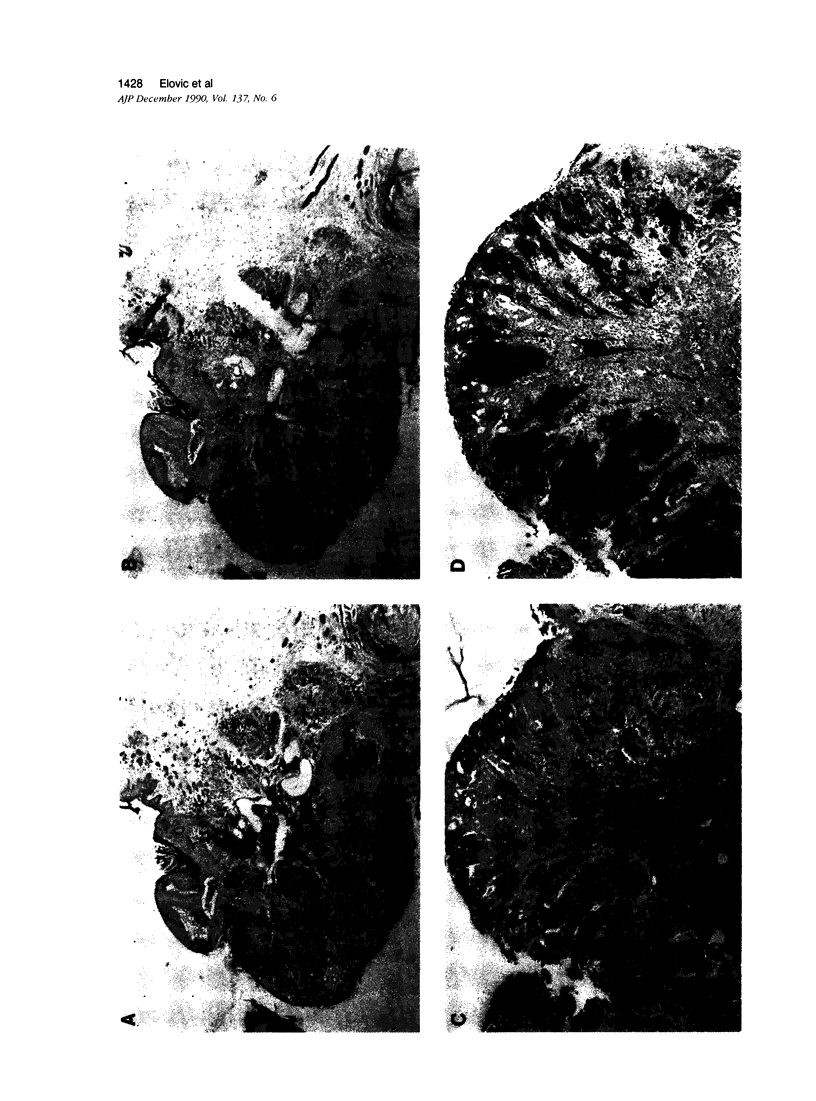
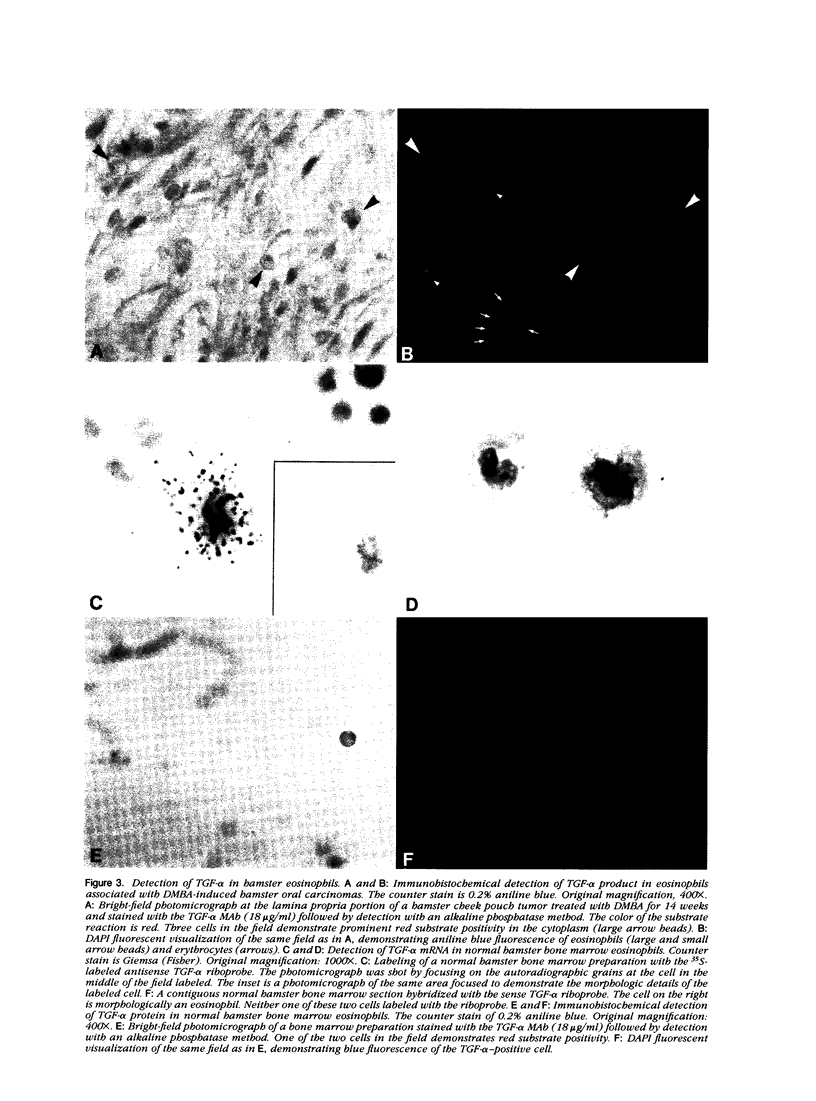
Previously it was demonstrated that malignant transformation of the Syrian hamster cheek pouchmucosa is associated with the expression of TGF-α. Therefore in situ hybridization and immunohistochemistry was used to investigate the cellular sources of TGF-α production in this model system. Surprisingly one cell type in the inflammatory infiltrate present in the connective tissue adjacent to the transformed epithelium represented a major source of TGF-α mRNA. Detailed analysis of these cells revealed that they were eosinophils. In addition to TGF-α mRNA, about 40% of the eosinophils associated with the oral tumors exhibited TGF-α product reactive with a monoclonal antibody against the C terminus of the mature TGF-α peptide. Normal hamster bone marrow eosinophils also exhibited TGF-α mRNA and product by in situ hybridization and immunohistochemistry. These results suggest that the eosinophil represents a biologically significant source of TGF-α.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anklesaria P., Teixidó J., Laiho M., Pierce J. H., Greenberger J. S., Massagué J. Cell-cell adhesion mediated by binding of membrane-anchored transforming growth factor alpha to epidermal growth factor receptors promotes cell proliferation. Proc Natl Acad Sci U S A. 1990 May;87(9):3289–3293. doi: 10.1073/pnas.87.9.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann R., Lindquist P. B., Nagashima M., Kohr W., Lipari T., Napier M., Derynck R. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell. 1989 Feb 24;56(4):691–700. doi: 10.1016/0092-8674(89)90591-6. [DOI] [PubMed] [Google Scholar]
- Chang L. C., Chou M. Y., Chow P., Matossian K., McBride J., Tao C. A., Gallagher G. T., Wong D. T. Detection of transforming growth factor-alpha messenger RNA in normal and chemically transformed hamster oral epithelium by in situ hybridization. Cancer Res. 1989 Dec 1;49(23):6700–6707. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Derynck R., Goeddel D. V., Ullrich A., Gutterman J. U., Williams R. D., Bringman T. S., Berger W. H. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987 Feb 1;47(3):707–712. [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Fox C. H., Kotler D., Tierney A., Wilson C. S., Fauci A. S. Detection of HIV-1 RNA in the lamina propria of patients with AIDS and gastrointestinal disease. J Infect Dis. 1989 Mar;159(3):467–471. doi: 10.1093/infdis/159.3.467. [DOI] [PubMed] [Google Scholar]
- Gordon J. R., Galli S. J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990 Jul 19;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Chang C. K., Posnett D. N., Fanelli B., Tam J. P. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988 Feb 1;167(2):670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han V. K., Hunter E. S., 3rd, Pratt R. M., Zendegui J. G., Lee D. C. Expression of rat transforming growth factor alpha mRNA during development occurs predominantly in the maternal decidua. Mol Cell Biol. 1987 Jul;7(7):2335–2343. doi: 10.1128/mcb.7.7.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrin M. S., Samsoondar J., Kudlow J. E. Alpha-transforming growth factor secreted by untransformed bovine anterior pituitary cells in culture. II. Identification using a sequence-specific monoclonal antibody. J Biol Chem. 1986 Nov 5;261(31):14414–14419. [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- McCrone E. L., Lucey D. R., Weller P. F. Fluorescent staining for leukocyte chemotaxis. Eosinophil-specific fluorescence with aniline blue. J Immunol Methods. 1988 Nov 10;114(1-2):79–88. doi: 10.1016/0022-1759(88)90157-3. [DOI] [PubMed] [Google Scholar]
- Patterson S., Gross J., Webster A. D. DNA probes bind non-specifically to eosinophils during in situ hybridization: carbol chromotrope blocks binding to eosinophils but does not inhibit hybridization to specific nucleotide sequences. J Virol Methods. 1989 Feb;23(2):105–109. doi: 10.1016/0166-0934(89)90124-9. [DOI] [PubMed] [Google Scholar]
- Plaut M., Pierce J. H., Watson C. J., Hanley-Hyde J., Nordan R. P., Paul W. E. Mast cell lines produce lymphokines in response to cross-linkage of Fc epsilon RI or to calcium ionophores. Nature. 1989 May 4;339(6219):64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Shklar G. Experimental oral pathology in the Syrian hamster. Prog Exp Tumor Res. 1972;16:518–538. doi: 10.1159/000393387. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Chiu I. M., Pan C. J., Cohn R. H., Kedes L. H., Marzluff W. F. Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4078–4082. doi: 10.1073/pnas.78.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Wodnar-Filipowicz A., Heusser C. H., Moroni C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature. 1989 May 11;339(6220):150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- Wong D. T., Gallagher G. T., Gertz R., Chang A. L., Shklar G. Transforming growth factor alpha in chemically transformed hamster oral keratinocytes. Cancer Res. 1988 Jun 1;48(11):3130–3134. [PubMed] [Google Scholar]
- Wong D. T., Weller P. F., Galli S. J., Elovic A., Rand T. H., Gallagher G. T., Chiang T., Chou M. Y., Matossian K., McBride J. Human eosinophils express transforming growth factor alpha. J Exp Med. 1990 Sep 1;172(3):673–681. doi: 10.1084/jem.172.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. T., Winchell L. F., McCune B. K., Earp H. S., Teixidó J., Massagué J., Herman B., Lee D. C. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell. 1989 Feb 10;56(3):495–506. doi: 10.1016/0092-8674(89)90252-3. [DOI] [PubMed] [Google Scholar]
- Zeller R., Bloch K. D., Williams B. S., Arceci R. J., Seidman C. E. Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev. 1987 Sep;1(7):693–698. doi: 10.1101/gad.1.7.693. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]