Abstract
The occlusion derived form of baculovirus is specially adapted for primary infection of the host midgut epithelium. As such, the virion must contain the proteins essential for host range determination and initiation of infection. Because knowledge of virion composition is a prerequisite for functional investigation, this study used a combination of techniques to identify the proteins present within or associated with the occlusion-derived virus (ODV) virion. Thirty-one proteins, including proteins known to be essential for viral DNA replication, were identified with confidence. An additional 13 proteins were identified by using one of the three techniques. A comparison of gene conservation among the ODV proteins encoded in the 16 sequenced baculoviridae genomes is presented. With knowledge of the composition of ODV, it is now possible to target proteins and study their role(s) during primary infection.
Autographa californica nucleopolyhedrovirus (AcMNPV) is the type species for the family Baculoviridae and it was used in this study. AcMNPV is a double-stranded DNA virus (132 kbp) that undergoes a biphasic life cycle in its lepidopteron host. Progeny nucleocapsids have two fates: during the early phase of infection, ≈16% of the intracellular copies of viral DNA are targeted for maturation at the cell surface to produce budded virus (BV) (1). The remaining nucleocapsids mature within the nucleus and are incorporated within a viral occlusion (occlusion-derived virus, ODV). After primary infection of the insect gut by ODV, BV is produced and released into the hemocoel, and secondary infection results in insect death with subsequent release of viral occlusions into the environment. Because ODV is the viral form responsible for primary infection, knowing virion composition is fundamental for functional investigation of virulence and host specificity. The goal of this study was to determine the protein composition of the ODV virion. Such knowledge should aid in the understanding of the biology of AcMNPV, including genetic manipulation of the family Baculoviridae to enhance their function as microbial pesticides (2). Additionally, studies on the mechanism of envelope protein trafficking to intranuclear membranes and ODV envelope would be aided by comprehensive knowledge of ODV envelope composition.
ODV is amenable to proteomic approaches for protein identification. It is easily purified and contains a small number of proteins. Previous studies suggest ODV contains between 13 and 35 proteins, most of which are unknown (3–13). ODV is incorporated within a crystalline occlusion, and the increased density of the occlusion allows it to be easily purified from in vitro or in vivo sources. A major concern when releasing the ODV from the crystalline matrix is protein degradation caused by the presence of proteases, particularly an insect alkaline protease (14). To inhibit protease activity, the occlusions are treated with HgCl2 or diisopropyl fluorophosphate (14). After protease inactivation, ODV is released from the occlusion and purified by using density-gradient centrifugation. When ODV is used as an antigen to produce polyclonal antibodies, cross-reactivity with lysates prepared from host cells is below detectable limits (12), suggesting that cellular protein contamination of ODV purified in this manner is minimal.
To comprehensively identify the protein composition of ODV, multiple approaches were used. To identify genes encoding epitopes uniquely presented in ODV, an expression library was screened by using antibodies generated to ODV or BV. Colonies positive only to ODV antiserum were isolated and the corresponding genes identified. MS was used in two ways. Peptide mass fingerprinting (PMF) is a sensitive protein identification technique that compares the masses of experimentally obtained peptides to theoretical digests of proteins available in databases (15). Because multiple peptide fragments are analyzed, protein identity can be determined even if various protein isoforms are present, or proteins are posttranslationally processed or partially degraded. Protein mixtures are typically separated by using 1D or 2D SDS/PAGE to simplify the MS analysis. More recently, multidimensional protein identification technology (MUDPIT) or “shotgun proteomics” has been developed (16). This technique differs from PMF in that the entire mixture is digested before separation and the resulting peptide mixture is processed by using 2D liquid chromatography and tandem MS (MS/MS). The peptide sequences are predicted from the observed CID mass spectra by comparison with published databases. After identification, proteins were chosen for verification of ODV localization if their function suggested significant insights into baculovirus biology, or if their presence within ODV disagreed with previous studies. When these techniques were used, 31 proteins were convincingly identified within ODV, including five proteins known to be essential for viral replication.
Materials and Methods
Cell Culture and Virus Purification. Spodoptera frugiperda (Sf9) cells were cultured at 27°C in complete TNMFH medium (17). AcMNPV (strain E2) infections were performed at a multiplicity of infection of 10. Budded virus was purified from the collected media as described in Summers and Smith (14). Occlusions were purified from infected Trichoplusia ni larvae and protease inactivation performed by using HgCl2 (14). ODV was released from the occlusions by using alkaline treatment and purified by using density gradient centrifugation and continuous sucrose gradients (12).
Expression Library Screening. A genomic AcMNPV library was prepared in λgt11 and was screened by using antisera prepared against BV and ODV. The details of the library construction and screen have been published (18). Fifty colonies unique to ODV were plaque purified, the DNA was rescued and sequenced, and the corresponding gene was determined. Library inserts initiating from different amino acids through the gene were considered independent isolates, and the number of independent isolates identifying each gene is indicated in Tables 1 and 2.
Table 1. Proteins locating to nucleocapsid and unknown localization.
| ORF | Identity | Library screen | SDS/PAGE, % coverage | MUDPIT-MS/MS | Western blot | Ref. |
|---|---|---|---|---|---|---|
| Identified by using multiple techniques | ||||||
| 9 | p78/83 | PKTAPETSTIVEVPTVLPK: LPPPAPSLSNVLSELK; SSTTNLIADVLADTINR | 34 | |||
| 49 | PCNA | 35 | ||||
| 54 | vp1054 | 36 | ||||
| 61 | FP25K | 8.8 | 37 | |||
| 65 | DNA polymerase | 8.6 | NDTQCANNTYKFCLYKMK | |||
| 66 | 93 kDa | 43.9* | LRQEFEIK; DENAERLSEIQLQK; SKLNTQLDELNSLFVK; QSVSIKDQEIAM; ESIADQAVKLLEQNQTDFESISEFISRDPAFNR | |||
| 67 | lef3 | X | ||||
| 70 | Hcf-1 | 1 | KENKEIYITSNK | |||
| 77 | Vlf-1 | 1 | 2.4* | 32 | ||
| 89 | p39 | 54.6 | RAVAPEYLQIDTEELR; RILIPSATNYQDVFNLNSM; NVLKFEGDTQR; VLPIFDEDDNQFK; NDFIPR; YTEGFTSTTQR; AVAPEYLQIDTEELR; FFDVTNAR; VIHSVYATTK; GGAGDQLFNNYSGFLQNLIR | 38 | ||
| 95 | Helicase | KLENVV | X | |||
| 100 | p6.9 | 37.5 | SSTGTTYGSTRR | 39 | ||
| 101 | BV/ODV-C42 | TTLEELLIER | 40 | |||
| 102 | 13.1 kDa | 23.6* | ILSTQSVGAR: LQTINTAASQTAASLLINDITPNKTESLK | |||
| 104 | p87 | 48.2 | SAEDDLLPTR | 41 | ||
| 109 | 44.8 kDa | 34.5* | IIFPYQLVPNVIIK; TNINFVTQR; FFAFPHNLVEPQSDVGNK; QAQSLLGIPDYSQTVVDFVK; PDFSPPNTFDYSDYANR | |||
| 114 | 49 kDa | ETIVNIINSYHNACQNLK; LAALDFIILM; NYVQPAIVNLFESHNR; IINELLFLNDNVNYATNK | ||||
| 129 | p24 | 42 | ||||
| 132 | 25.1 kDa | 41.1 | AIAAEQTLR | |||
| 142 | 49 kDa | 58.3* | GLPLFK; DLNPWVQNTLLK; NAFYAPK; ILSILAVNR; SKYTVVNSTK; YDHESSYIFYSK; LTGDVYVVDKNEK; YIKPGTPVYATNLFTSNPR; SGGGNLLTLERDHFK | |||
| 144 | ODV-EC27 | 2 | TYELAEFDLK; TVTEIVNSDEKIQK | 43 | ||
| 147 | IE1 | X | ||||
| Identified by using one technique | ||||||
| 5 | 12.4 kDa | ITEYVGDVK; INNAPVVASQHDYDRDQIKR | ||||
| 14 | lef1 | 2 | ||||
| 22 | 44 kDa | 29.6 | ||||
| 30 | 54.5 kDa | 1 | ||||
| 39 | p43 | 8.5 | ||||
| 58 | 6.8 | SKKFPIGEVVSTR | ||||
| 59 | 8.2 kDa | LFVETFTK | ||||
| 74 | 30.5 kDa | 1 | ||||
| 79 | 12.1 kDa | 2 | ||||
| 86 | PNK/PNL | 6.3 | ||||
| 88 | cg30 | VESLHFNVYSVNRNVVDVIK | ||||
| 92 | p33 | IPLTPLFSR | ||||
| 114 | 49 kDa | ETIVNIINSYHNACQNLK; LAALDFIILM; NYVQPAIVNLFESHNR; IINELLFLNDNVNYATNK | ||||
| 133 | Alk-exo | 1.8%* | ||||
Boldface indicates previously identified components of ODV.
Includes sequences determined by using MS/MS; see Tables 4 and 5 for a full description.
Table 2.
Envelope proteins
| ORF | Identity | Library screen | SDS/PAGE, % coverage | MUDPIT-MS/MS | Western blot | Ref. |
|---|---|---|---|---|---|---|
| 16 | BV-ODV-E26 | 44 | ||||
| 23 | f-protein | 1 | X | |||
| 46 | ODV-E66 | 2 | 17.9* | NGTLYSNVIGNFIFYPAVHSADYSK; LNVEGHSDSLR;; QNNIQELQNFER; YWLGLYLPTAVNSM; VIVLSR; IPSGTTSTQSFRPTIGQTAIAK; YNNTSDTLYQNPELAYNLINGLR; TYYGSVVGVTNRNITIVLNETQHYDEAASLTR; TDTAGAILVYAK | 45 | |
| 80 | gp41 | 6 | 46.8* | DANAIIAAAAPNATRPNTR; ILFINTIR; FIFQQINYNK; HATLPPNIQSAVESR; SNSTNSVIAPYNK; LGKDALAEAAK; ILFINTIRDM; FQNATFLTSAANAVNSPAAHLTK; CNDMSELSPLMILFINTIRDM | 46 | |
| 83 | p91 | 1 | 47 | |||
| 94 | ODV-E25 | 3 | 46.5* | GAANFDIK; VANLR; LSQVYIAEKPLSIDDIVK; IAHGDNKLSQVYIAEKPLSIDDIVK; VGTNSVFLGTVYDYGIK | 48 | |
| 49 | ||||||
| 138 | p74 | 12.6* | 50 | |||
| 143 | ODV-E18 | 54.8 | 43 | |||
| 148 | ODV-E56 | 3 | 46.2* | 18, 51 |
Boldface indicates previously identified components of ODV.
Includes sequences determined by using MS/MS; see Tables 4 and 5 for a full description.
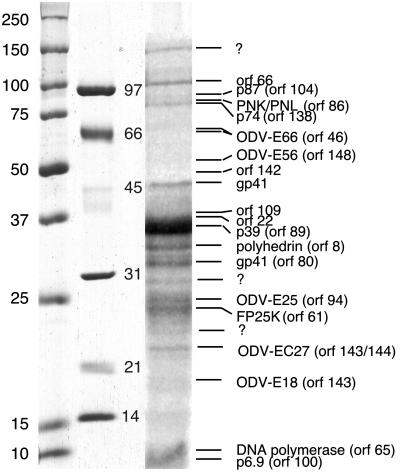
MS. SDS/PAGE and in-gel digestion. The proteins of ODV (30 μg) were separated by using SDS/PAGE (19). The separated proteins were subjected to in-gel trypsin digestion and analyzed by using a matrix assisted laser desorption/ionization time-of-flight MS (20). Bands that were not confidently identified or appeared to consist of multiple proteins were subjected to MS/MS using a PE-Sciex Qstar Pulsar quadrupole time-of-flight. A detailed protocol is included in Appendix 1, which is published as supporting information on the PNAS web site, www.pnas.org. A total of four gels were analyzed in this manner, with a sample gel shown in Fig. 1.
Fig. 1.
ODV proteins were separated on a 7–15% gradient gel and stained with Coomassie blue. The bands were subjected to in-gel trypsin, and their determined identity is listed to the right. Those bands marked with a question mark either did not produce significant peptides for analyses, or the peptide masses did not match predicted peptides from the AcMNPV genome. Prestained and unstained standards are used routinely; however, they vary significantly in the lower molecular weight range. As such both standards are included for reference [prestained, far left (Bio-Rad; precision); unstained, center (Bio-Rad, LMW)].
MUDPIT. ODV (300 μg) was processed, and MS/MS was performed essentially as described (16). The detailed protocol is reported in Appendix 1.
Western Blot Analysis. ODV, BV, and Sf9 cells were collected, and proteins were separated by using SDS/PAGE and transferred to poly(vinylidene difluoride) (PVDF). The PVDF was treated with primary antibody, washed, treated with appropriate secondary IgG-HRP, and washed. Target proteins were detected by using chemiluminescence. Orf23 protein antibodies were generated to amino acids 95–370 fused to glutatione S-transferase. Protein was produced by induction in bacteria and purified by using SDS/PAGE, and the antiserum was pretreated with purified glutatione S-transferase to remove IgGs generated to the fusion portion of the clone.
Results
A summary of ODV associated proteins is presented in Tables 1 and 2. Proteins previously confirmed as components of ODV are shown in boldface in Table 1. A comprehensive compilation of the data are presented in Tables 4 and 5, which are published as supporting information on the PNAS web site.
Expression Library Screen. Fifty colonies positive only with ODV antiserum were isolated, the library insert was sequenced, and the number of independent isolates are noted in Tables 1 and 2. Seven identified ORFs corresponded to known structural proteins: ODV-EC27, vlf-1, ODV-E66, ODV-E56, ODV-E25, gp41, and p96. This screen also identified hcf-1, orf30, lef1, orf74, orf79, and f-protein.
MS Identification of ODV Proteins. In the first approach, ODV proteins were separated by using SDS/PAGE (Fig. 1), and bands were excised and trypsin digested. The AcMNPV-encoded peptides identified in this study are shown in Tables 4 and 5 (SDS/PAGE; matrix assisted laser desorption/ionization), and the data are summarized in Fig. 1 and in Tables 1 and 2 (SDS/PAGE; percent coverage). This approach identified the proteins FP25K, DNA polymerase, orf66, orf77, p39, p6.9, orf102, p87, orf109, orf132, orf142, orf22, and p43 (Table 1) and the envelope proteins ODV-E66, gp41, ODV-E25, p74, ODV-E18, and ODV-E56 (Table 2). The data from some of the bands suggested that additional proteins could be present. The digested peptides from these bands were subjected to MS/MS, and this analyses identified orf66, vlf-1, orf102, orf109, orf142, alkaline-exonuclease, ODV-E66, gp41, ODV-E25, ODV-E56, and p74 (asterisk in Tables 1, 2, 4, and 5). Three bands did not identify an AcMNPV encoded protein (question mark in Fig. 1). It is possible that these bands represent cellular proteins.
MUDPIT (16) was also used to identify ODV proteins. In this approach, proteins are immediately denatured and subjected to chemical cleavage and trypsin digestion. The MUDPIT analysis identified p78/83, DNA polymerase, orf66, hcf-1, p39, helicase, p6.9, BV/ODV-C42, orf102, p87, orf109, orf114, orf132, orf142, ODV-EC27, orf5, orf58, orf59, cg30, p33, alkaline-exonuclease (Table 1); and the envelope proteins ODV-E66, gp41, and ODV-E25 (Table 2). This coverage is reported in Tables 4 and 5. It is possible that peptides present in low concentrations may be hidden among more highly represented peptide species and were missed using this technique.
Verification of Protein Localization to ODV. A number of proteins were identified by using more than one technique, thus providing a high degree of confidence that these proteins are present in ODV. These proteins include DNA polymerase, orf66, hcf-1, vlf-1, p39, p6.9, orf102, p87, orf109, orf132, orf142, ODV-EC27, and the envelope proteins ODV-E66, gp41, ODV-E25, and ODV-E56. Several proteins known to be associated with ODV were identified by one technique: p78/83, FP25K, BV/ODV-C42, p91, p74, and ODV-E18 (Tables 1 and 2). For further analyses, our attention was directed to proteins identified by one technique and with the following characteristics: (i) protein detection within ODV conflicts with previous studies; or (ii) proteins with a function that would suggest an important aspect of AcMNPV biology.
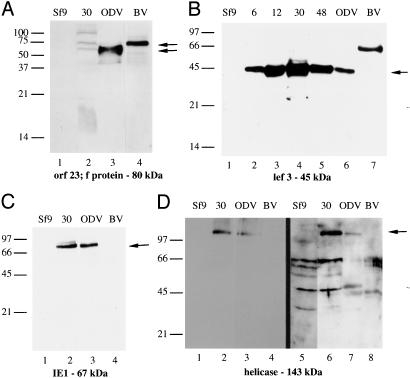
Previous data reports that f-protein is BV-specific (21), however our results suggest that f-protein is also present in ODV. To test this, antiserum was generated to f-protein and used to probe infected cell lysates and purified virus. In purified ODV, a band at ≈75 kDa was detected (Fig. 2A, lane 3). The positive band detected in BV has a higher Mr (Fig. 2 A, lane 4). Because the detection of f-protein with ODV conflicts with published data (21), various ODV purification protocols were tested to determine whether purification conditions affect detection. F-protein was detected only when HgCl2 was used to inhibit proteases during ODV purification; if this treatment was omitted, f-protein was not detected (data not shown).
Fig. 2.
(A) Sf9 and 30 h postinfection (hpi) infected cell lysates, ODV and BV probed with antibody to orf23 protein (catalog no. 8787; 1:5,000). (B) Total cell lysates from Sf9 cells (lane 1) and cells infected with AcMNPV collected through a temporal time course (hpi, lanes 2–5), purified ODV (lane 6), and BV (lane 7) were probed with antibody to lef3 (1:5,000). The 46-kDa immunoreactive protein is noted with an arrow. (C) Lysates from Sf9 cells and infected cells collected at 30 hpi, purified ODV, and BV were probed with antibody to IE1 (arrow; 1:5,000). (D) Lysates from Sf9 cells and cells collected at 30 hpi, purified ODV and BV were probed with monoclonal (1:500, lanes 1–4) and polyclonal (1:2,000, lanes 5–8) antibodies to helicase. The immunoreactive band of helicase is noted with an arrow. All cell lysates are loaded at 15 μg per lane, and ODV and BV were loaded at 10 μg per lane.
ODV Contains Proteins Essential for Viral DNA Replication. Six viral proteins are essential for viral DNA replication: helicase, DNA polymerase, IE1, lef1, lef2, and lef3 (22). In this study lef1, lef2, helicase, and DNA polymerase were identified in ODV. Because their presence within ODV suggests important biological functions, we used Western blot to confirm their association with ODV. It is possible that the other essential viral DNA replication proteins are also present within ODV, although at levels precluding their identification by MS methods. As such, these were also included in our study. Antiserum was not available for lef1, lef2, or DNA polymerase; however, Western blot analysis was performed by using antibodies to lef3, helicase, and IE1. Time course analysis was performed for all of these proteins; however, IE1 and helicase did not show a size shift between the protein detected in the temporal analysis and that incorporated into viral progeny. As such, the temporal analysis is only shown for lef3. Lef3 is detected at the predicted molecular mass (45 kDa) in infected cell lysates and in purified ODV (Fig. 2B, lanes 1–6); however, a protein migrating at a larger molecular mass is detected in BV (≈67 kDa; Fig. 2B, lane 7). IE1 was detected within purified ODV but not in BV (Fig. 2C). This result is in conflict with published results (23), so, as performed previously, various ODV purification conditions were tested to determine whether they affected IE1 detection. Protease inactivation using HgCl2 during the purification of ODV was essential for detection of IE1 (data not shown). We did not detect IE1 in BV; however, it has been detected within BV purified from cells infected with OpMNPV (23). Helicase was detected within ODV but not within BV (Fig. 2D).
Conservation of Genes Encoding Proteins Associated with ODV. When the sequences and annotated genomes of Baculoviridae are compared, a large number of the genes encoding ODV-associated proteins are conserved (Table 3). The genes for gp41, p74, ODV-E56, orf22, orf23, vp1054, vlf-1, p39, orf92, p6.9, orf109, orf133, orf142, ODV-EC27, lef1, DNA polymerase, and helicase are present in the genomes of the sequences baculoviruses except BuSuNPV, where only a partial genome is available. Phylogenetic analyses show that CuniNPV (Culex nigripalpus) may represent a new genus distinct from Nucleopolyhedrovirus or Granulovirus (24). If the CuniNPV genome is excluded, the genes for ODV-E66, ODV-E25, ODV-E18, FP25K, ODV-C42, lef3, and IE1 are also conserved (Table 3). A few genes are not conserved: BV/ODV-E26, PCNA, hcf-1, PNK/PNL, orf5, orf30, orf39, orf58, orf79, orf114, and orf132.
Table 3. Conservation of ORFs (assigned orf number) among annotated baculovirus genomes.
| AcMNPV | Identity | BmMNPV | OpMNPV | LdMPV | SeMNPV | HaSNPV | HzSNPV | SpitMNPV | EppoMNPV | MacoNPV | RoMNPV | CuniNPV | BusuNPV | CpGV | PhopGV | XcGV | PxGV | Other baculoviruses |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ODV envelope proteins | ||||||||||||||||||
| 16 | BV/ODV-E26 | 8 | 15 | - | - | - | - | - | 13 | - | 14 | - | - | - | - | - | ||
| 22* | 44 kDa | 13 | 20 | 119 | 35 | 132 | 136 | 135 | 18 | 48 | 19 | 38 | 48 | 44 | 45 | 37 | CfMNPV, NeSeNPV | |
| 23* | f-protein | 14 | 21 | 130 | 8 | 133 | 137 | 136 | 19 | 9 | 20 | 104 | 31 | 27 | 27 | 26 | ||
| 46† | ODV-E66 | 37 | 50 | 131 | 57/114 | 96 | 99 | 98 | 42 | 78/144 | 43 | - | 37 | 33 | 149 | 30 | LsNPV, CfMNPV | |
| 80* | gp41 | 66 | 83 | 88 | 80 | 73 | 75 | 76 | 72 | 104 | 77 | 33 | 25 | 104 | 97 | 121 | 87 | AgMNPV, SfNPV |
| 83* | p96/vp91 | 69 | 86 | 91 | 77 | 76 | 78 | 79 | 75 | 101 | 80 | 35 | 101 | 94 | 118 | 84 | ||
| 94† | ODV-E25 | 77 | 95 | 96 | 71 | 82 | 85 | 85 | 82 | 94 | 91 | - | 91 | 83 | 99 | 74 | LsNPV, TnGV | |
| 138* | p74 | 115 | 134 | 27 | 131 | 20 | 19 | 21 | 121 | 160 | 131 | 74 | 60 | 55 | 77 | 49 | LsNPV, CfMNPV, TnNPV, SlNPV | |
| 143† | ODV-E18 | 119 | 140 | 19 | 136 | 10 | 10 | 12 | 125 | 166 | 135 | - | 44 | 14 | 12 | 12 | 13 | CfGV |
| 148* | ODV-E56 | 124 | 146 | 14 | 6 | 15 | 15 | 17 | 130 | 6 | 140 | 102 | 51 | 18 | 16 | 15 | 16 | CfMNPV, AsGV |
| Nucleocapsid and unassigned location proteins | ||||||||||||||||||
| 5 | 12.4 kDa | 134 | 7 | - | - | - | - | - | 5 | - | 3 | - | - | - | - | - | AfNPV | |
| 9 | p78/83 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 136 | - | 7 | - | - | 2 | 2 | - | AgMNPV, AfNPV | |
| 30 | 54.6 kDa | 21 | 38 | - | - | - | - | 30 | 30 | - | 27 | - | - | - | - | - | ||
| 39 | p43 | 30 | - | - | - | - | - | - | - | - | 36 | - | - | - | - | - | ||
| 49 | PCNA | - | 53 | - | - | - | - | - | - | - | 46 | - | - | - | - | - | ||
| 54* | vp 1054 | 43 | 58 | 57 | 105 | 47 | 48 | 49 | 49 | 133 | 52 | 8 | 34 | 138 | 126 | 175 | 115 | |
| 58 | 6.8 kDa | 47 | - | - | - | - | - | - | - | - | 56 | - | - | - | - | - | ||
| 59 | 8.2 kDa | 47 | 62 | 61 | 101 | 51 | 52 | 52 | - | - | 56 | - | 36 | - | - | - | - | LsNPV |
| 61† | FP25K | 49 | 64 | 63 | 98 | 53 | 54 | 57 | 55 | 125 | 58 | - | 39 | 118 | 110 | 140 | 100 | |
| 66 | 93 kDa | 54 | 71 | 82 | 92 | 66 | 68 | 68 | 59 | 114 | 63 | - | - | - | 133 | 94 | ||
| 70 | hcf-1 | - | - | - | - | - | - | - | - | - | 67 | - | - | - | - | 22 | ||
| 74 | 30.5 kDa | 60 | 77 | 135 | - | 68 | 70 | 71 | 66 | - | 71 | - | - | - | - | - | ||
| 77* | vlf-1 | 63 | 80 | 86 | 82 | 71 | 73 | 74 | 69 | 106 | 74 | 18 | 27 | 106 | 99 | 123 | 89 | AgMNPV |
| 79 | 12.1 kDa | 65 | 82 | - | - | - | - | - | 71 | - | 76 | - | 65 | - | 75 | - | AgMNPV | |
| 86 | PNK/PNL | - | - | - | - | - | - | - | - | - | 83 | - | - | - | - | - | ||
| 88 | cg30 | 71 | 89 | - | 76 | 77 | 80 | 80 | 76 | - | 85 | - | - | - | - | - | ||
| 89* | p39 | 72 | 90 | 92 | 75 | 78 | 81 | 81 | 77 | 99 | 86 | 24 | 96 | 88 | 111 | 79 | ||
| 92* | p33 | 75 | 93 | 94 | 73 | 80 | 83 | 83 | 80 | 96 | 89 | 14 | 93 | 85 | 101 | 76 | LsNPV | |
| 100* | p6.9 | 84 | 101 | 101 | 65 | 88 | 91 | 90 | 87 | 86 | 96 | 23 | 86 | 78 | 94 | 67 | ||
| 101† | BV/ODV-C42 | 85 | 102 | 102 | 64 | 89 | 92 | 91 | 88 | 85 | 97 | - | 85 | 77 | 93 | 66 | ClGV | |
| 102 | 13.1 kDa | 86 | 103 | 103 | 63 | 90 | 93 | 92 | 89 | 84 | - | - | - | 92 | 64 | |||
| 104 | vp80/p87 | 88 | 105 | 105 | 61 | 92 | 95 | 94 | 91 | - | 100 | - | - | - | - | - | CfMNPV, LsNPV | |
| 109* | 44.8 kDa | 92 | 109 | 107 | 59 | 94 | 97 | 96 | 95 | 80 | 104 | 69 | 55 | 50 | 53 | 43 | LsNPV | |
| 114 | 49 kDa | 94 | 114 | - | - | - | - | - | - | - | 108 | - | - | - | - | - | ||
| 129 | p24 | 106 | 127 | - | 10 | 118 | 122 | 116 | 114 | - | 122 | - | 11 | 71 | 63 | 80 | 53 | CfMNPV, HcNPV |
| 132 | 25.1 kDa | 109 | 130 | - | - | - | - | - | 117 | - | 125 | - | - | - | - | - | CfMNPV | |
| 133* | alk exo | 110 | 131 | 157 | 41 | 114 | 117 | 109 | 118 | 54 | 126 | 54 | 125 | 114 | 145 | 106 | CfMNPV | |
| 142* | 49 kDa | 118 | 139 | 20 | 137 | 9 | 9 | 11 | 124 | 167 | 134 | 30 | 15 | 13 | 13 | 14 | CfGV | |
| 144* | ODV-EC27 | 120 | 141 | 18 | 135 | 11 | 11 | 13 | 126 | 165 | 136 | 32 | 45 | 97 | 89 | 112 | 80 | |
| Viral DNA replication proteins | ||||||||||||||||||
| 14* | lef1 | 6 | 13 | 123 | 14 | 124 | 128 | 129 | 11 | 35 | 12 | 45 | 3 | 74 | 66 | 82 | 55 | CfMNPV |
| 65* | DNA polymerase | 53 | 70 | 83 | 93 | 67 | 69 | 61 | 58 | 115 | 62 | 91 | yes | 111 | 103 | 132 | 93 | CfMNPV, SlNPV, MbNPV, OaNPV |
| 67† | lef3 | 55 | 72 | 81 | 91 | 65 | 67 | 67 | 60 | 113 | 64 | - | 113 | 105 | 134 | 95 | SlNPV | |
| 95* | Helicase | 78 | 96 | 97 | 70 | 84 | 87 | 866 | 73 | 93 | 92 | 89 | 24 | 90/126 | 82/113 | 98 | 72 | |
| 147† | IE1 | 123 | 145 | 15 | 132 | 14 | 14 | 16 | 129 | 162 | 139 | - | 46 | 7 | 6 | 9 | 10 | TnSNPV, CfMNPV, HcNPV |
Virus acronym description and complete reference list are provided in Appendix 2, which is published as supporting information on the PNAS web site.
ORF is conserved among all sequenced baculovirus genomes.
ORF is conserved among all genomes except CuniNPV.
Discussion
The importance of using multiple approaches to identify the proteins associated with AcMNPV ODV is demonstrated by this study. MUDPIT identified 24 baculovirus proteins as components of ODV, providing the largest number of proteins of the three approaches used. Ten of these proteins were identified only with MUDPIT. SDS/PAGE followed by either matrix assisted laser desorption/ionization time-of-flight (MALDI-TOF) MS or ESI quadrupole time-of-flight (qTOF) MS identified 21 proteins, 7 of which were only identified with this technique. Whether the differences in protein identification are caused by differences in separation techniques, ionization techniques, or mass spectrometer (TOF/qTOF vs. ion trap) is unknown, but similar results have been reported (B. L. Allen, personal communication). The library screen identified 13 proteins, 6 of which were not identified by the MS techniques. Proteins not identified by using SDS/PAGE may be due to low copy number, resistance to staining, or they are not amenable to MALDI-TOF MS. Other factors, such as protein susceptibility to degradation or losses caused by purification, can also affect protein detection. For instance, as part of the purification procedure, ODV samples were solubilized with the nonionic detergent, Nonidet P-40. This treatment can result in a loss of membrane-associated proteins, and ODV envelope and envelope-associated proteins may be underrepresented. Additionally, the occlusion proteins polyhedrin and pp34 were commonly identified by using MUDPIT (data not shown), suggesting that they are present in abundance and may have masked low abundant proteins. Protease degradation may also complicate protein identification. For example, DNA polymerase was convincingly identified from a band migrating below 10 kDa, even though its predicted molecular mass is 114 kDa. DNA polymerase was identified from a single sequence by MUDPIT. It is possible that DNA polymerase is sensitive to degradation and nonspecific cleavages from naturally occurring proteases. This would result in peptides not recognized by database searching. Even when multiple approaches were used, four proteins identified in previous studies (PCNA, vp1054, p24, and BV/ODV-E26) were not identified here. Clearly, the use of multiple techniques is essential if the goal is complete proteome determination.
Both IE1 and f-protein were identified associated with ODV. This was unexpected because previous studies suggest that these proteins are present only in BV (23, 25–27). Neither IE1 nor f-protein was detected in ODV if HgCl2 treatment of occlusions was omitted. This was true even if mammalian protease inhibitors were used throughout purification. Thus, mammalian protease inhibitors are not a substitute for HgCl2 treatment. It is likely these proteins are susceptible to degradation. These two examples demonstrate the fragile nature of some proteins and the variability associated with different protocols for virus purification.
The results of this study suggest that five of the six proteins essential for replication of viral DNA are present in ODV: DNA polymerase, helicase, IE1, lef3, and lef1. It is possible that lef2 is also present, but antibodies were not available to directly test it. Although DNA polymerase activity has been detected within the inner core of the hepatitis B virus (28), we have described the incorporation of almost a complete set of essential viral DNA replication proteins within a virion. Several possibilities can be postulated: (i) these proteins may serve a function during viral assembly and maturation; (ii) they may be incorporated into ODV as a consequence of protein abundance or colocalization. We note however, that other proteins such as pp31 or p10 that are also in high concentrations in areas of viral assembly, were not identified. Thus, although random incorporation cannot be excluded, it seems likely that viral assembly proceeds with a high degree of specificity. (iii) AcMNPV may incorporate these proteins in ODV for reasons related to primary infection and viral DNA replication. Within the hostile environment of the gut, primary infection must include viral attachment and entry into gut cells, rapid replication, and production of progeny BV. It is possible that AcMNPV has evolved mechanisms to optimize progeny virus production in these difficult conditions. Even before viral gene expression, the presentation of these proteins by parental virus may facilitate the initiation of viral DNA replication. The seminal studies identifying the essential replication proteins were performed by using transient expression of viral genes in vitro to determine their ability to support plasmid replication (22, 29). It is possible that the mechanism used by ODV during primary infection of gut cells has significant differences from the mechanism postulated by the results of in vitro assays. There is evidence that, during infection, other proteins participate in viral DNA replication: lef11 may be required (30), and hcf-1 exerts cell-line-specific effects on viral DNA replication rates (31). Vlf-1 may also be involved in this process: it is essential for productive infection, and based upon homology, it may function as a topoisomerase, resolvase (32), or integrase/recombinase. The functional role for the replication proteins within ODV will only be revealed when studies are specifically directed to elucidate the mechanisms involved at the time of primary infection.
There was no attempt in this study to quantitate the amount of each protein present within ODV. It is not clear that such knowledge would reveal significant insights. Even if a protein is in low abundance, low copy number does not imply insignificant function. ODV may contain 1–20 genomes per enveloped virion, so even if protein copy number per genome equivalent was determined, protein copy numbers per virion would still be unknown. Mass spectrometers are inherently poor quantitative devices. Current analytical instrumentation can qualitatively distinguish 3 to 4 orders of magnitude difference in protein concentration, yet in relation to each other, cellular proteins can easily range from 6 to 10 orders of magnitude in concentration (33). The variance in protein concentration for AcMNPV ODV is unknown. It is possible that proteins present in low abundance were not detected by this analysis.
The goal of this study was to identify proteins associated with ODV. It is possible that ODV contains host proteins; however, no special effort was directed at identifying them. The unidentified SDS/PAGE separated protein bands may reflect the presence of such proteins, but in the absence of a sequenced genome, it is unlikely that host genes would be identified. ODV comprises at least 31 proteins. Thirteen additional proteins were identified, and considering that the coverage of these proteins is significant or they were identified by more than one peptide sequence, it is highly likely they are present within ODV. Thus, these data suggest that ODV contains between 31 and 44 proteins. This number compares favorably to, but is larger than, earlier reports that suggest that ODV contains 13–35 unique proteins.
With knowledge of the composition of ODV, it is now possible to target relevant proteins for studies of function and the elucidation of their potential role(s) during primary infection.
Supplementary Material
Acknowledgments
We thank L. A. Guarino for antibody to IE1, G. F. Rohrmann for polyclonal antibodies to lef3 and helicase, and E. Carstens for monoclonal antibody to helicase. We thank Debra Elton for her analyses of the expression library screen and Jessica Lucas for help with analysis of the MS data. This study was supported by Texas Agricultural Experiment Station Project TEX08078 (M.D.S.), National Science Foundation Grants DBI-0116685 and CHE-9629966, and Department of Energy Grant DE-FG03-9ER14505 (to D.H.R.).
Abbreviations: AcMNPV, Autographa californica nucleopolyhedrovirus; ODV, occlusion-derived virus; BV, budded virus; MS/MS, tandem MS; MUDPIT, multidimensional protein identification technology.
References
- 1.Rosinski, M., Reid, S. & Nielsen, L. K. (2002) Biotechnol. Bioeng. 77, 476–480. [PubMed] [Google Scholar]
- 2.Smith, C. R., Heinz, K. M., Sansone, C. G. & Flexner, J. L. (2000) Biol. Control 19, 201–214. [Google Scholar]
- 3.Summers, M. D. & Smith, G. E. (1978) Virology 84, 390–402. [DOI] [PubMed] [Google Scholar]
- 4.Smith, G. E. & Summers, M. D. (1981) J. Virol. 39, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knell, J. D., Summers, M. D. & Smith, G. E. (1983) Virology 125, 381–392. [DOI] [PubMed] [Google Scholar]
- 6.Monroe, J. E. & McCarthy, W. J. (1984) J. Invertebr. Pathol. 43, 32–40. [Google Scholar]
- 7.Maruniak, J. E. (1979) Ph.D. dissertation (Univ. of Texas, Austin).
- 8.Stiles, B. & Wood, H. A. (1983) Virology 131, 230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maruniak, J. E. & Summers, M. D. (1981) Virology 109, 25–34. [DOI] [PubMed] [Google Scholar]
- 10.Maskos, C. B. & Miltenburger, H. G. (1981) J. Invertebr. Pathol. 37, 174–180. [Google Scholar]
- 11.Vlak, J. M. (1979) J. Invertebr. Pathol. 34, 110–118. [Google Scholar]
- 12.Braunagel, S. C. & Summers, M. D. (1994) Virology 202, 315–328. [DOI] [PubMed] [Google Scholar]
- 13.Singh, S. P., Gudauskas, R. Y., Harper, J. D. & Edwards, J. (1985) J. Invertebr. Pathol. 45, 249–251. [Google Scholar]
- 14.Summers, M. D. & Smith, G. E. (1975) J. Virol. 16, 1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aebersold, R. & Goodlett, D. R. (2002) Chem. Rev. 101, 269–295. [DOI] [PubMed] [Google Scholar]
- 16.Washburn, M. P., Wolters, D. & Yates, J. R. (2001) Nat. Biotechnol. 19, 242–247. [DOI] [PubMed] [Google Scholar]
- 17.Summers, M. D. & Smith, G. E. (1987) A Manual of Methods for Baculovirus Vectors and Insect Cell Culture Procedures (Texas Agricultural Experiment Station, College Station), Bulletin 1555.
- 18.Braunagel, S. C., Elton, D. M., Ma, H. & Summers, M. D. (1996) Virology 217, 97–110. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. (1970) Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 20.Mortz, E., Vorm, O., Mann, M. & Roepstorff, P. (1994) Biol. Mass Spectrom. 23, 249–261. [DOI] [PubMed] [Google Scholar]
- 21.Pearson, M. N., Russell, R. L. Q. & Rohrmann, G. F. (2001) Virology 291, 22–31. [DOI] [PubMed] [Google Scholar]
- 22.Kool, M., Ahrens, C. H., Goldbach, R. W., Rohrmann, G. F. & Vlak, J. M. (1994) Proc. Natl. Acad. Sci. USA 91, 11212–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theilmann, D. A. & Stewart, S. (1993) J. Gen. Virol. 74, 1819–1826. [DOI] [PubMed] [Google Scholar]
- 24.Moser, B. S., Becnel, J. J., White, S. E., Afonso, C. L., Kutish, G. F., Shanker, S. & Almira, E. (2001) J. Gen. Virol. 82, 283–297. [DOI] [PubMed] [Google Scholar]
- 25.Pearson, M. N., Groten, C. & Rohrmann, G. F. (2000) J. Virol. 74, 6126–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westenberg, M., Wang, H., Ijkel, W. F. J., Goldbach, R. W., Vlak, J. M. & Zuidema, D. (2002) J. Virol. 76, 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ijkel, W. F. J., Westenberg, M., Goldbach, R. W., Blissard, G. W., Vlak, J. M. & Zuidema, D. (2000) Virology 275, 30–41. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan, P. M., Greenman, R. L., Gerin, J. L., Purcell, R. H. & Robinson, W. S. (1973) J. Virol. 12, 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, A. & Miller, L. K. (1995) J. Virol. 69, 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, G. & Blissard, G. W. (2002) J. Virol. 76, 2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu, A. & Miller, L. K. (1996) J. Virol. 70, 5123–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang, S. & Miller, L. K. (1998) Virology 245, 99–109. [DOI] [PubMed] [Google Scholar]
- 33.Patterson, S. D. (2003) Nat. Biotechnol. 21, 221–222. [DOI] [PubMed] [Google Scholar]
- 34.Pearson, M. N., Russell, R. L., Rohrmann, G. F. & Beaudreau, G. S. (1988) Virology 167, 407–413. [PubMed] [Google Scholar]
- 35.Belyavskyi, M., Braunagel, S. C. & Summers, M. D. (1998) Proc. Natl. Acad. Sci. USA 95, 11205–11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olszewski, J. & Miller, L. K. (1997) J. Virol. 71, 5040–5050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braunagel, S. C., Burks, J. K., Rosas-Acosta, G., Harrison, R. L., Ma, H. & Summers, M. D. (1999) J. Virol. 73, 8559–8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiem, S. M. & Miller, L. K. (1989) J. Virol. 63, 2008–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson, M. E. & Price, K. H. (1991) J. Invertebr. Pathol. 57, 264–268. [Google Scholar]
- 40.Braunagel, S. C., Guidry, P. A., Rosas-Acosta, G., Engelking, L. & Summers, M. D. (2001) J. Virol. 75, 12331–12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu, A. & Carstens, E. B. (1992) Virology 190, 201–209. [DOI] [PubMed] [Google Scholar]
- 42.Wolgamot, G. M., Gross, C. H., Russell, R. L. & Rohrmann, G. F. (1993) J. Gen. Virol. 74, 103–107. [DOI] [PubMed] [Google Scholar]
- 43.Braunagel, S. C., He, H., Ramamurthy, P. & Summers, M. D. (1996) Virology 222, 100–114. [DOI] [PubMed] [Google Scholar]
- 44.Beniya, H., Braunagel, S. C. & Summers, M. D. (1998) Virology 240, 64–75. [DOI] [PubMed] [Google Scholar]
- 45.Hong, T., Braunagel, S. C. & Summers, M. D. (1994) Virology 204, 210–222. [DOI] [PubMed] [Google Scholar]
- 46.Whitford, M. & Faulkner, P. (1992) J. Virol. 66, 4763–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell, R. L. Q., Funk, C. J. & Rohrmann, G. F. (1997) Virology 227, 142–152. [DOI] [PubMed] [Google Scholar]
- 48.Hong, T., Summers, M. D. & Braunagel, S. C. (1997) Proc. Natl. Acad. Sci. USA 94, 4050–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russell, R. Q. & Rohrmann, G. F. (1993) Virology 195, 532–540. [DOI] [PubMed] [Google Scholar]
- 50.Kuzio, J., Jaques, R. & Faulkner, P. (1989) Virology 173, 759–763. [DOI] [PubMed] [Google Scholar]
- 51.Theilmann, D. A., Chandler, J. K., Stewart, S., Flipsen, H. T. M., Vlak, J. M. & Crooks, N. E. (1996) Virology 218, 148–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.