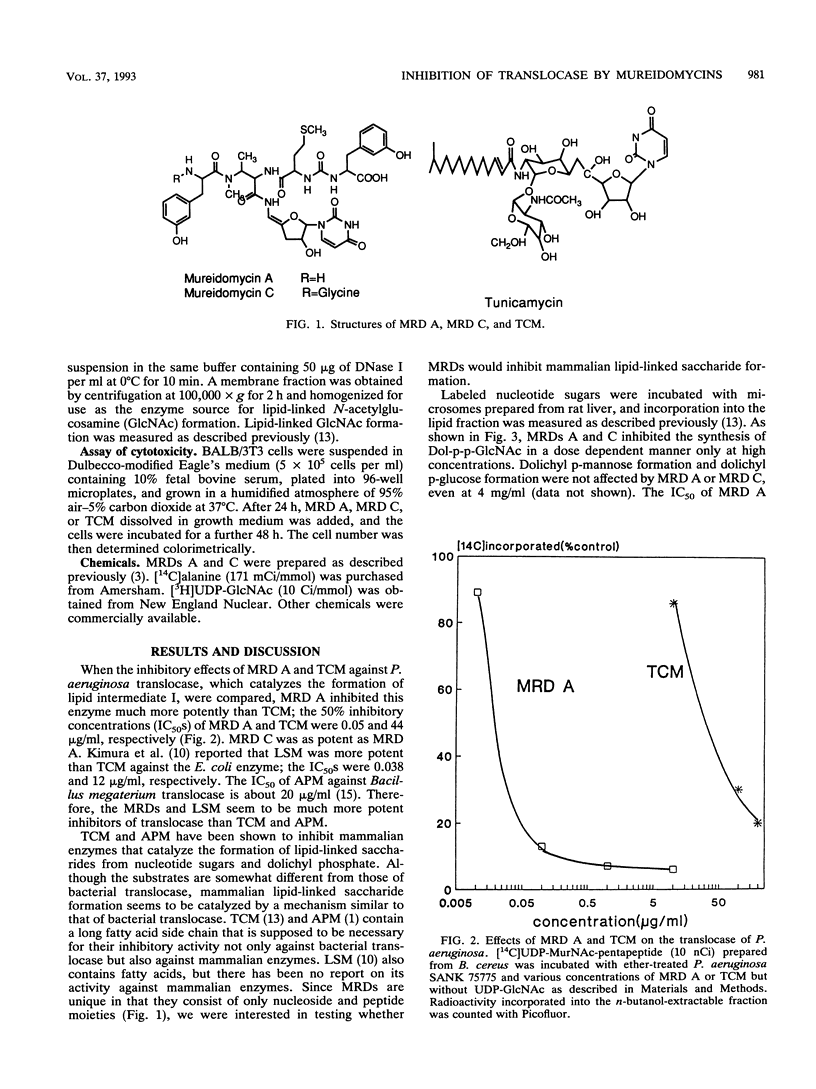
Abstract
Mureidomycins (MRDs) A and C inhibited strongly the formation of undecaprenyl pyrophosphoryl N-acetylmuramyl-pentapeptide (lipid intermediate I), which is an intermediate in bacterial peptidoglycan synthesis (50% inhibitory concentration [IC50] of MRD A, 0.05 microgram/ml). However, they did not inhibit the formation of dolichyl pyrophosphoryl N-acetylglucosamine (Dol-p-p-GlcNAc), dolichyl phosphoryl glucose, or dolichyl phosphoryl mannose, the precursors for mammalian glycoprotein synthesis, or the formation in Bacillus subtilis of lipid-linked N-acetylglucosamine for teichoic acid synthesis (IC50s, > 100 micrograms/ml). In contrast, tunicamycin (TCM) inhibited strongly the formation of Dol-p-p-GlcNAc (IC50, 0.03 microgram/ml) but inhibited weakly the formation of bacterial lipid intermediate I (IC50, 44 micrograms/ml). When the effects of MRDs A and C and TCM on the growth of mammalian cells were compared, MRDs did not show any toxicity, even at 1,000 micrograms/ml, whereas TCM inhibited the growth of BALB/3T3 cells at 10 micrograms/ml. On the basis of these results, it was concluded that MRDs are the first specific and potent inhibitors of the translocase reaction in bacterial peptidoglycan synthesis, showing a high level of toxicity against bacteria and a low level of toxicity against mammalian cells. A specific inhibitor of translocase could be a potent antibiotic with highly selective toxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodanszky M., Sigler G. F., Bodanszky A. Structure of the peptide antibiotic amphomycin. J Am Chem Soc. 1973 Apr 4;95(7):2352–2357. doi: 10.1021/ja00788a040. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Inukai M., Isono F., Takahashi S., Enokita R., Sakaida Y., Haneishi T. Mureidomycins A-D, novel peptidylnucleoside antibiotics with spheroplast forming activity. I. Taxonomy, fermentation, isolation and physico-chemical properties. J Antibiot (Tokyo) 1989 May;42(5):662–666. doi: 10.7164/antibiotics.42.662. [DOI] [PubMed] [Google Scholar]
- Isono F., Inukai M. Mureidomycin A, a new inhibitor of bacterial peptidoglycan synthesis. Antimicrob Agents Chemother. 1991 Feb;35(2):234–236. doi: 10.1128/aac.35.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isono F., Inukai M., Takahashi S., Haneishi T., Kinoshita T., Kuwano H. Mureidomycins A-D, novel peptidylnucleoside antibiotics with spheroplast forming activity. II. Structural elucidation. J Antibiot (Tokyo) 1989 May;42(5):667–673. doi: 10.7164/antibiotics.42.667. [DOI] [PubMed] [Google Scholar]
- Isono F., Katayama T., Inukai M., Haneishi T. Mureidomycins A-D, novel peptidylnucleoside antibiotics with spheroplast forming activity. III. Biological properties. J Antibiot (Tokyo) 1989 May;42(5):674–679. doi: 10.7164/antibiotics.42.674. [DOI] [PubMed] [Google Scholar]
- Isono F., Kodama K., Inukai M. Susceptibility of Pseudomonas species to the novel antibiotics mureidomycins. Antimicrob Agents Chemother. 1992 May;36(5):1024–1027. doi: 10.1128/aac.36.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. S., Spencer J. P., Elbein A. D. Amphomycin inhibition of mannose and GlcNAc incorporation into lipid-linked saccharides. J Biol Chem. 1978 Dec 25;253(24):8860–8866. [PubMed] [Google Scholar]
- Kang M. S., Spencer J. P., Elbein A. D. Amphomycin inhibits the incorporation of mannose and GlcNAc into lipid-linked saccharides by aorta extracts. Biochem Biophys Res Commun. 1978 May 30;82(2):568–574. doi: 10.1016/0006-291x(78)90912-9. [DOI] [PubMed] [Google Scholar]
- Oka T. Mode of action of penicillins in vivo and in vitro in Bacillus megaterium. Antimicrob Agents Chemother. 1976 Oct;10(4):579–591. doi: 10.1128/aac.10.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsuki A., Arima K., Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 1971 Apr;24(4):215–223. doi: 10.7164/antibiotics.24.215. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Oiwa R., Matsukura S., Omura S. Amphomycin inhibits phospho-N-acetylmuramyl-pentapeptide translocase in peptidoglycan synthesis of Bacillus. Biochem Biophys Res Commun. 1979 Feb 14;86(3):902–908. doi: 10.1016/0006-291x(79)91797-2. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]


