Abstract
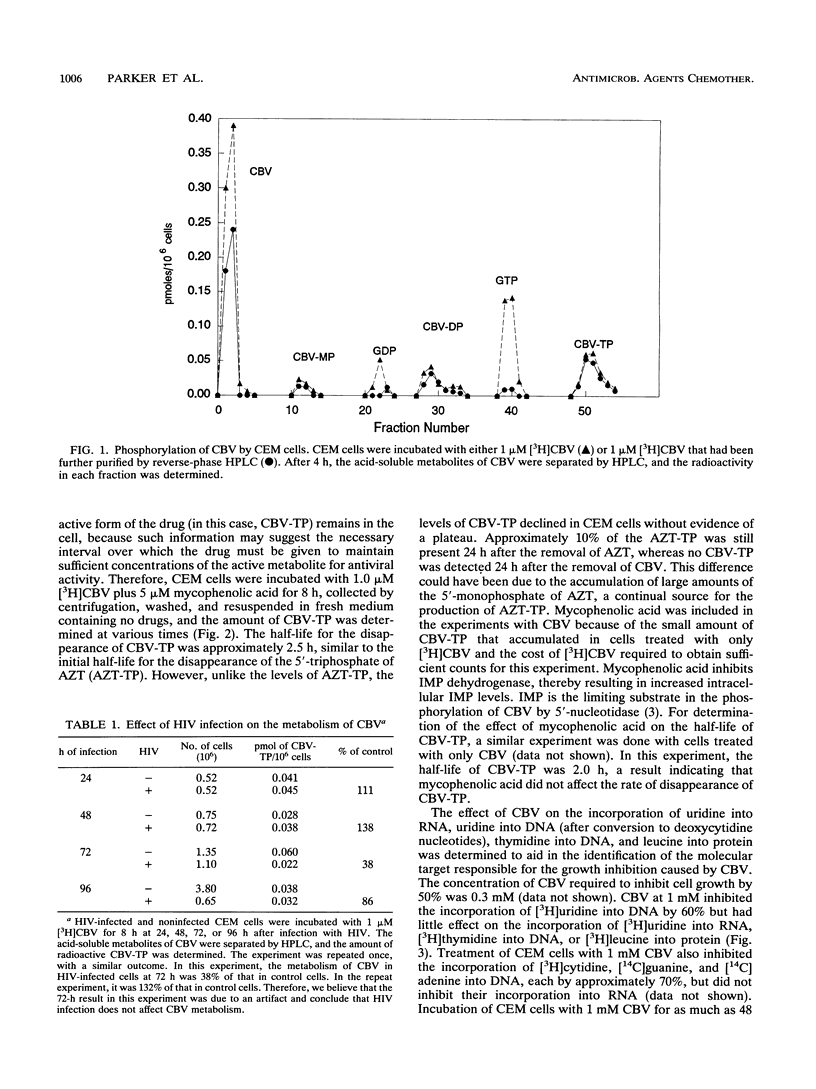
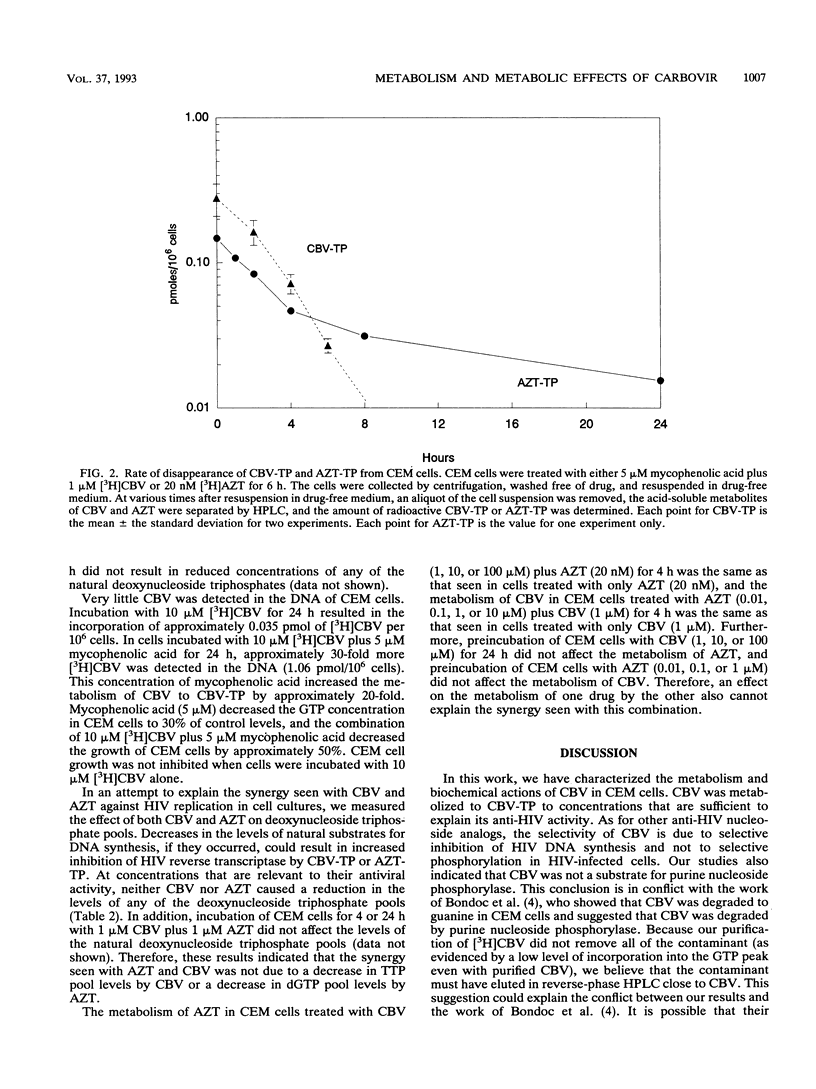
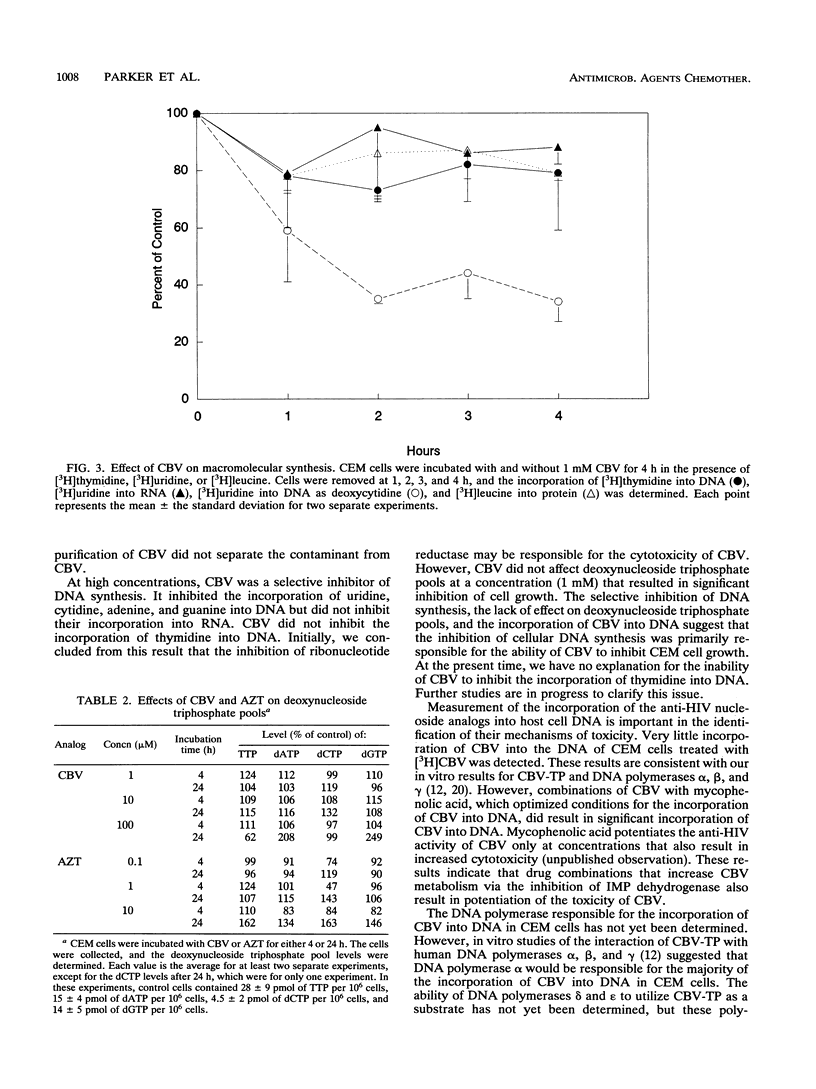
Carbovir (CBV) [the (--)-enantiomer of the carbocyclic analog of 2',3'-dideoxy-2',3'-didehydroguanosine] is a potent inhibitor of human immunodeficiency virus type 1 (HIV) replication in vitro. We have characterized the metabolism of CBV and its effect on cellular metabolism in an effort to better understand its mechanism of action. CBV was primarily metabolized to the 5'-triphosphate of CBV (CBV-TP) to concentrations sufficient to inhibit HIV reverse transcriptase. Infection of CEM cells with HIV did not affect the metabolism of CBV. In CEM cells, there was no evidence of the degradation of CBV by purine nucleoside phosphorylase. The half-life of CBV-TP in CEM cells was 2.5 h, similar to that of the 5'-triphosphate of zidovudine (AZT). However, unlike the levels of the 5'-triphosphate of AZT, CBV-TP levels declined without evidence of a plateau. CBV did not affect the metabolism of AZT, and AZT did not affect the metabolism of CBV. A small amount of CBV was incorporated into DNA in intact CEM cells, and this incorporation was increased by incubation with mycophenolic acid, an inhibitor of IMP dehydrogenase. CBV specifically inhibited the incorporation of nucleic acid precursors into DNA but had no effect on the incorporation of radiolabeled precursors into RNA or protein. CBV did not decrease the level of TTP, dGTP, dCTP, or dATP. These results suggested that the cytotoxicity of CBV was due to the inhibition of DNA synthesis. Further studies are necessary to identify the target(s) responsible for growth inhibition.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avramis V. I., Markson W., Jackson R. L., Gomperts E. Biochemical pharmacology of zidovudine in human T-lymphoblastoid cells (CEM). AIDS. 1989 Jul;3(7):417–422. doi: 10.1097/00002030-198907000-00002. [DOI] [PubMed] [Google Scholar]
- Bennett L. L., Jr, Smithers D., Hill D. L., Rose L. M., Alexander J. A. Biochemical properties of the nucleoside of 3-amino-1,5-dihydro-5-methyl-1,4,5,6,8-pentaazaacenaphthylene (NSC-154020). Biochem Pharmacol. 1978 Jan 15;27(2):233–241. doi: 10.1016/0006-2952(78)90306-4. [DOI] [PubMed] [Google Scholar]
- Bondoc L. L., Jr, Shannon W. M., Secrist J. A., 3rd, Vince R., Fridland A. Metabolism of the carbocyclic nucleoside analogue carbovir, an inhibitor of human immunodeficiency virus, in human lymphoid cells. Biochemistry. 1990 Oct 23;29(42):9839–9843. doi: 10.1021/bi00494a013. [DOI] [PubMed] [Google Scholar]
- Carter S. G., Kessler J. A., Rankin C. D. Activities of (-)-carbovir and 3'-azido-3'-deoxythymidine against human immunodeficiency virus in vitro. Antimicrob Agents Chemother. 1990 Jun;34(6):1297–1300. doi: 10.1128/aac.34.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates J. A., Inggall H. J., Pearson B. A., Penn C. R., Storer R., Williamson C., Cameron J. M. Carbovir: the (-) enantiomer is a potent and selective antiviral agent against human immunodeficiency virus in vitro. Antiviral Res. 1991 Feb;15(2):161–168. doi: 10.1016/0166-3542(91)90033-n. [DOI] [PubMed] [Google Scholar]
- Du D. L., Volpe D. A., Grieshaber C. K., Murphy M. J., Jr In vitro toxicity of 3'-azido-3'-deoxythymidine, carbovir and 2',3'-didehydro-2',3'-dideoxythymidine to human and murine haematopoietic progenitor cells. Br J Haematol. 1992 Apr;80(4):437–445. doi: 10.1111/j.1365-2141.1992.tb04555.x. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Fridland A. Phosphorylation of 2',3'-dideoxyinosine by cytosolic 5'-nucleotidase of human lymphoid cells. Mol Pharmacol. 1989 Aug;36(2):291–295. [PubMed] [Google Scholar]
- Kurtzberg J., Carter S. G. Differential toxicity of carbovir and AZT to human bone marrow hematopoietic progenitor cells in vitro. Exp Hematol. 1990 Nov;18(10):1094–1096. [PubMed] [Google Scholar]
- Parker W. B., Shaddix S. C., Chang C. H., White E. L., Rose L. M., Brockman R. W., Shortnacy A. T., Montgomery J. A., Secrist J. A., 3rd, Bennett L. L., Jr Effects of 2-chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine on K562 cellular metabolism and the inhibition of human ribonucleotide reductase and DNA polymerases by its 5'-triphosphate. Cancer Res. 1991 May 1;51(9):2386–2394. [PubMed] [Google Scholar]
- Parker W. B., White E. L., Shaddix S. C., Ross L. J., Buckheit R. W., Jr, Germany J. M., Secrist J. A., 3rd, Vince R., Shannon W. M. Mechanism of inhibition of human immunodeficiency virus type 1 reverse transcriptase and human DNA polymerases alpha, beta, and gamma by the 5'-triphosphates of carbovir, 3'-azido-3'-deoxythymidine, 2',3'-dideoxyguanosine and 3'-deoxythymidine. A novel RNA template for the evaluation of antiretroviral drugs. J Biol Chem. 1991 Jan 25;266(3):1754–1762. [PubMed] [Google Scholar]
- Solter A. W., Handschumacher R. E. A rapid quantitative determination of deoxyribonucleoside triphosphates based on the enzymatic synthesis of DNA. Biochim Biophys Acta. 1969 Feb 18;174(2):585–590. doi: 10.1016/0005-2787(69)90288-3. [DOI] [PubMed] [Google Scholar]
- Sommadossi J. P., Carlisle R., Zhou Z. Cellular pharmacology of 3'-azido-3'-deoxythymidine with evidence of incorporation into DNA of human bone marrow cells. Mol Pharmacol. 1989 Jul;36(1):9–14. [PubMed] [Google Scholar]
- Starnes M. C., Cheng Y. C. Cellular metabolism of 2',3'-dideoxycytidine, a compound active against human immunodeficiency virus in vitro. J Biol Chem. 1987 Jan 25;262(3):988–991. [PubMed] [Google Scholar]
- Vazquez-Padua M. A., Starnes M. C., Cheng Y. C. Incorporation of 3'-azido-3'-deoxythymidine into cellular DNA and its removal in a human leukemic cell line. Cancer Commun. 1990;2(1):55–62. doi: 10.3727/095535490820874740. [DOI] [PubMed] [Google Scholar]
- Vince R., Brownell J. Resolution of racemic carbovir and selective inhibition of human immunodeficiency virus by the (-) enantiomer. Biochem Biophys Res Commun. 1990 May 16;168(3):912–916. doi: 10.1016/0006-291x(90)91115-9. [DOI] [PubMed] [Google Scholar]
- Vince R., Hua M., Brownell J., Daluge S., Lee F. C., Shannon W. M., Lavelle G. C., Qualls J., Weislow O. S., Kiser R. Potent and selective activity of a new carbocyclic nucleoside analog (carbovir: NSC 614846) against human immunodeficiency virus in vitro. Biochem Biophys Res Commun. 1988 Oct 31;156(2):1046–1053. doi: 10.1016/s0006-291x(88)80950-1. [DOI] [PubMed] [Google Scholar]
- White E. L., Parker W. B., Macy L. J., Shaddix S. C., McCaleb G., Secrist J. A., 3rd, Vince R., Shannon W. M. Comparison of the effect of Carbovir, AZT, and dideoxynucleoside triphosphates on the activity of human immunodeficiency virus reverse transcriptase and selected human polymerases. Biochem Biophys Res Commun. 1989 Jun 15;161(2):393–398. doi: 10.1016/0006-291x(89)92611-9. [DOI] [PubMed] [Google Scholar]


