Abstract
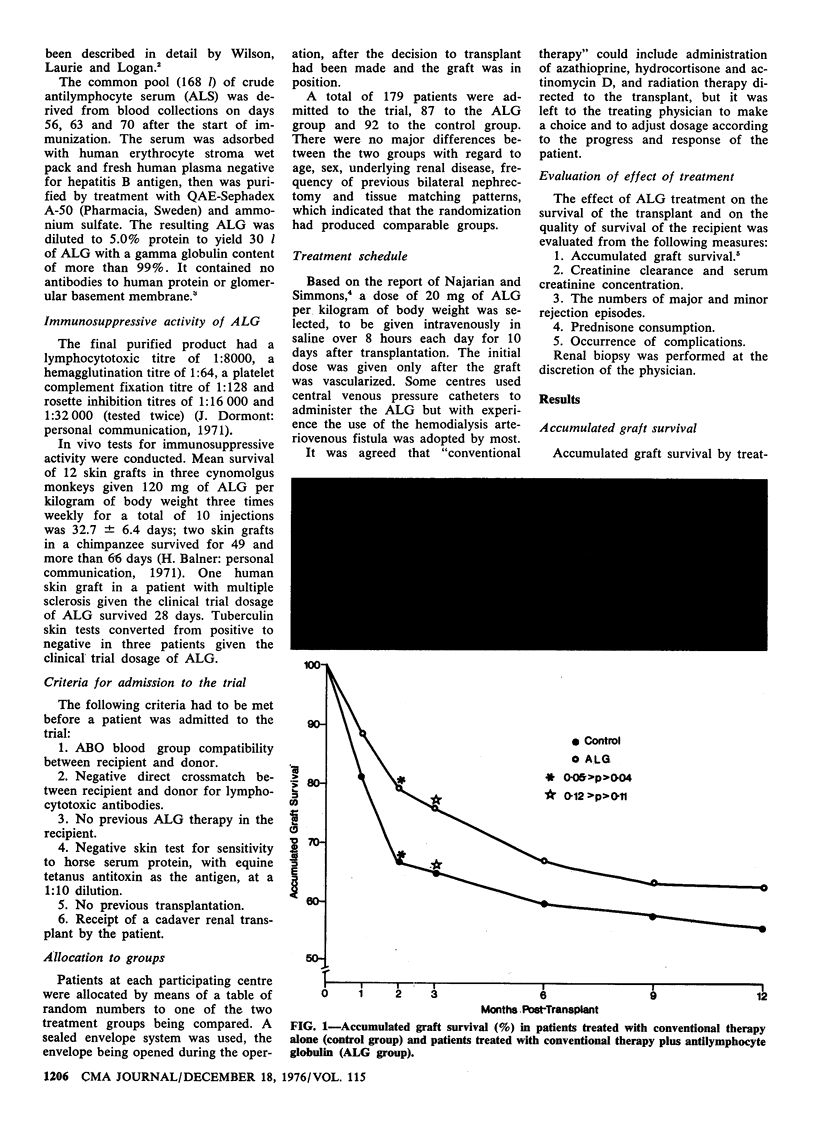
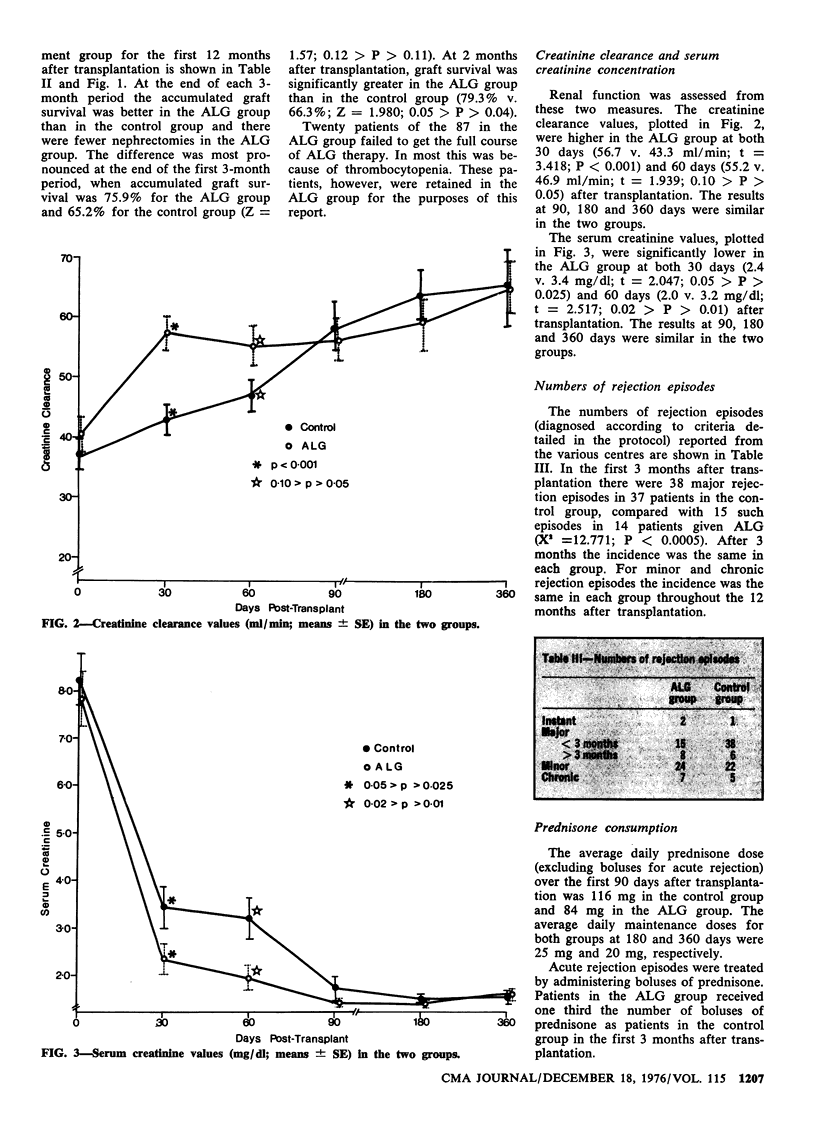
A multicentre, randomized clinical trial of antilymphocyte globulin (ALG) was conducted among patients who had undergone cadaver kidney transplantation; follow-up was continued for a minimum of 1 year. Of the 179 patients 92 were given conventional treatment only, while 87 were given in addition ALG (from a standardized, highly immunosuppressive, common pool of equine ALG), 20 mg/kg-d intravenously for 10 days after transplantation. The ALG-treated group had better accumulated graft survival, fewer nephrectomies, better graft function, less than half the number of acute rejection episodes and less prednisone use. There was a beneficial drug (ALG)-related effect in both the graft and the host during the first 3 months after transplantation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Najarian J. S., Simmons R. L. The clinical use of antilymphocyte globulin. N Engl J Med. 1971 Jul 15;285(3):158–166. doi: 10.1056/NEJM197107152850310. [DOI] [PubMed] [Google Scholar]
- Taylor H. E. The clinical application of antilymphocyte globulin. Med Clin North Am. 1972 Mar;56(2):419–432. doi: 10.1016/s0025-7125(16)32405-1. [DOI] [PubMed] [Google Scholar]
- Wilson S., Laurie G., Logan L. Standardized horse antihuman lymphocyte globulin for clinical use. Transplantation. 1973 Nov;16(5):466–475. doi: 10.1097/00007890-197311000-00011. [DOI] [PubMed] [Google Scholar]


