Abstract
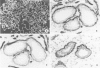
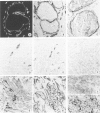
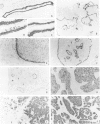
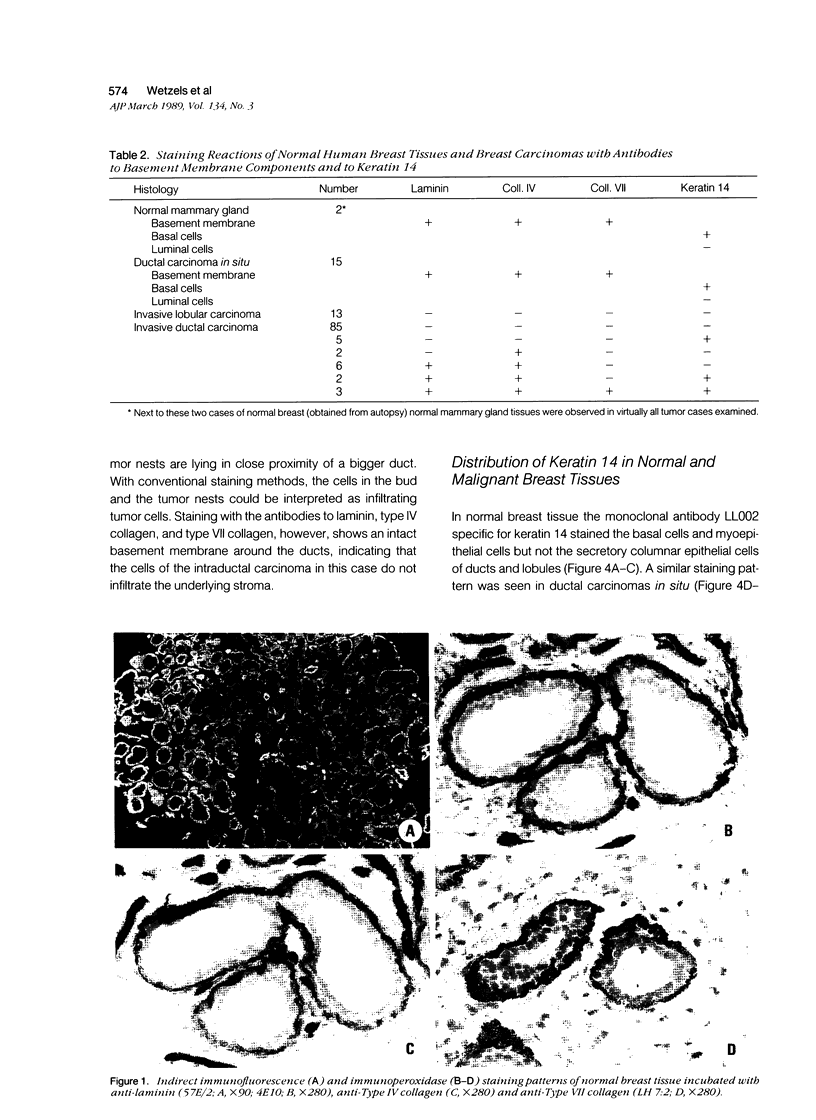
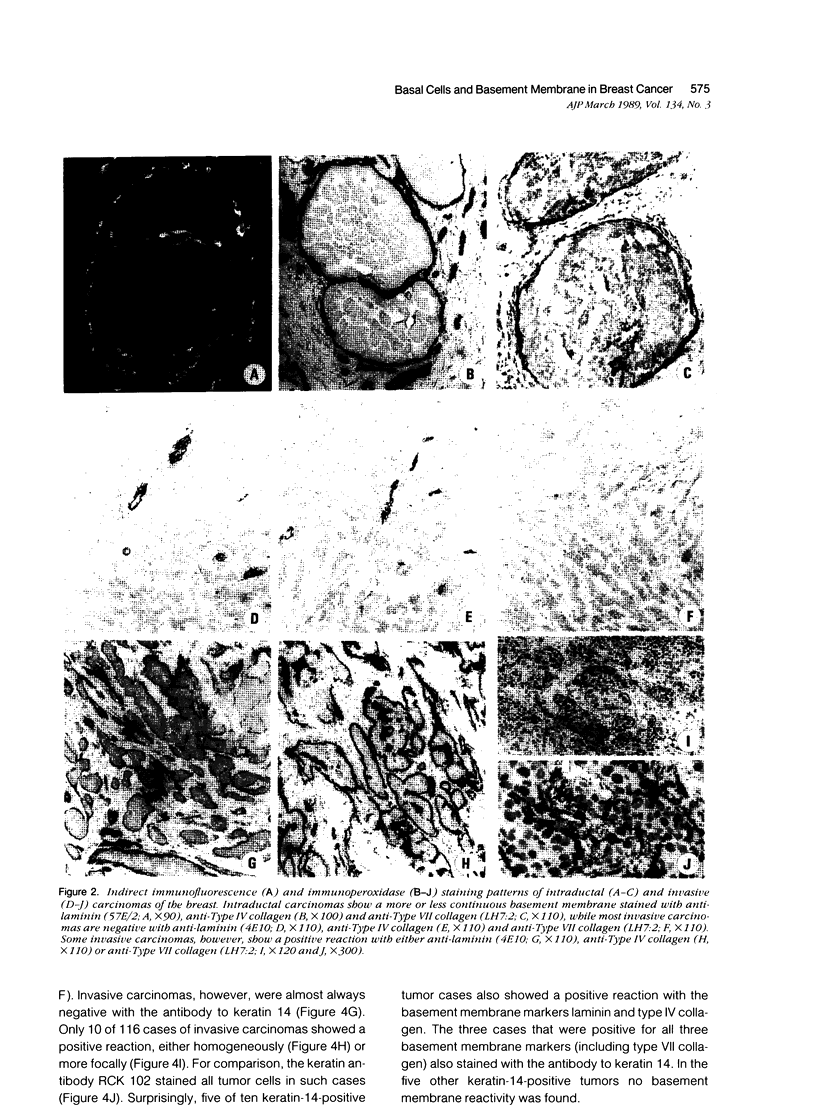
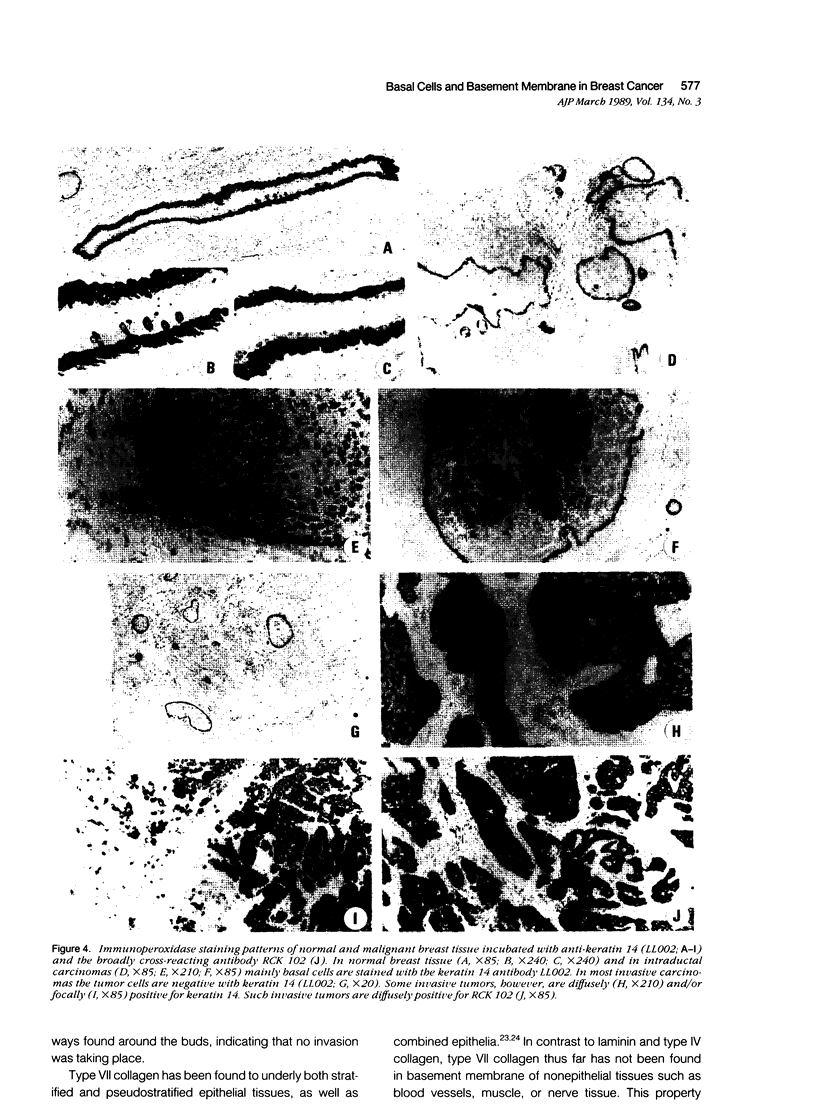
Using immunohistochemistry, the distribution patterns of basement membrane components type VII collagen (monoclonal antibody LH7.2), type IV collagen, and laminin were investigated in normal and malignant human breast tissue and compared with that of keratin 14 (monoclonal antibody LL002), which is expressed only by the basal (myoepithelial) cells in the secretory epithelia of the mammary gland. In normal breast tissue as well as in intraductal carcinomas, a more or less continuous basement membrane was observed at the epithelial stromal interface. Unlike laminin and type IV collagen, type VII collagen was not detected in the basement membrane of blood vessels. The keratin 14 antibody stained the basal cell layer of normal ducts and ducts with in situ cancer. In 85% of the invasive carcinomas no basement membrane or basal cells were detected. In 13 cases, however, laminin, type IV collagen, and/or type VII collagen were detected around tumor nests and individual tumor cells. Five of these tumors also showed a positive reaction with the keratin 14 antibody. In five cases keratin 14 expression was found without detectable basement membrane components. It is concluded that 18 of 103 invasive ductal breast carcinomas examined in this study exhibit a basal cell phenotype as determined from the expression of keratin and the deposition of basement membrane components.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R. Recent studies on the structure and pathology of basement membranes. J Pathol. 1986 Aug;149(4):257–278. doi: 10.1002/path.1711490402. [DOI] [PubMed] [Google Scholar]
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Bentz H., Morris N. P., Murray L. W., Sakai L. Y., Hollister D. W., Burgeson R. E. Isolation and partial characterization of a new human collagen with an extended triple-helical structural domain. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3168–3172. doi: 10.1073/pnas.80.11.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birembaut P., Caron Y., Adnet J. J., Foidart J. M. Usefulness of basement membrane markers in tumoural pathology. J Pathol. 1985 Apr;145(4):283–296. doi: 10.1002/path.1711450402. [DOI] [PubMed] [Google Scholar]
- Burtin P., Chavanel G., Foidart J. M., Martin E. Antigens of the basement membrane and the peritumoral stroma in human colonic adenocarcinomas: an immunofluorescence study. Int J Cancer. 1982 Jul 15;30(1):13–20. doi: 10.1002/ijc.2910300104. [DOI] [PubMed] [Google Scholar]
- Bussolati G. Actin-rich (myoepithelial) cells in lobular carcinoma in situ of the breast. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;32(2):165–176. doi: 10.1007/BF02889025. [DOI] [PubMed] [Google Scholar]
- Bussolati G., Botta G., Gugliotta P. Actin-rich (myoepithelial) cells in ductal carcinoma-in-situ of the breast. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;34(3):251–259. doi: 10.1007/BF02892422. [DOI] [PubMed] [Google Scholar]
- Charpin C., Lissitzky J. C., Jacquemier J., Lavaut M. N., Kopp F., Pourreau-Schneider N., Martin P. M., Toga M. Immunohistochemical detection of laminin in 98 human breast carcinomas: a light and electron microscopic study. Hum Pathol. 1986 Apr;17(4):355–365. doi: 10.1016/s0046-8177(86)80458-0. [DOI] [PubMed] [Google Scholar]
- Cooper D., Schermer A., Sun T. T. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab Invest. 1985 Mar;52(3):243–256. [PubMed] [Google Scholar]
- Dairkee S. H., Puett L., Hackett A. J. Expression of basal and luminal epithelium-specific keratins in normal, benign, and malignant breast tissue. J Natl Cancer Inst. 1988 Jul 6;80(9):691–695. doi: 10.1093/jnci/80.9.691. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Miettinen M., Forsman L., Andersson L. C. Basement membrane and apocrine epithelial antigens in differential diagnosis between tubular carcinoma and sclerosing adenosis of the breast. J Clin Pathol. 1984 Apr;37(4):357–363. doi: 10.1136/jcp.37.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E. R., Sass R., Fisher B., Wickerham L., Paik S. M. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol 6). I. Intraductal carcinoma (DCIS). Cancer. 1986 Jan 15;57(2):197–208. doi: 10.1002/1097-0142(19860115)57:2<197::aid-cncr2820570203>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Forster S. J., Talbot I. C., Critchley D. R. Laminin and fibronectin in rectal adenocarcinoma: relationship to tumour grade, stage and metastasis. Br J Cancer. 1984 Jul;50(1):51–61. doi: 10.1038/bjc.1984.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Ellison M., Neville A. M., Rudland P. S. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982 Nov;42(11):4763–4770. [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Mitchell D., Kraft N., Hancock W. W. Invading squamous cell carcinoma can retain a basal lamina. An immunohistochemical study using a monoclonal antibody to type IV collagen. Lab Invest. 1984 Jul;51(1):82–87. [PubMed] [Google Scholar]
- Gusterson B. A., Warburton M. J., Monaghan P., Foster C., Edwards P., Kraft N., Smith C., Mitchell D., Rudland P. S., Neville A. M. The use of immunohistochemical probes in the study of benign and malignant breast disease. Behring Inst Mitt. 1984 May;(74):39–48. [PubMed] [Google Scholar]
- Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161–220. [PubMed] [Google Scholar]
- Hancock W. W., Kraft N., Clarke F., Atkins R. C. Production of monoclonal antibodies to fibronectin, type IV collagen and other antigens of the human glomerulus. Pathology. 1984 Apr;16(2):197–206. doi: 10.3109/00313028409059105. [DOI] [PubMed] [Google Scholar]
- Jarasch E. D., Nagle R. B., Kaufmann M., Maurer C., Böcker W. J. Differential diagnosis of benign epithelial proliferations and carcinomas of the breast using antibodies to cytokeratins. Hum Pathol. 1988 Mar;19(3):276–289. doi: 10.1016/s0046-8177(88)80520-3. [DOI] [PubMed] [Google Scholar]
- Leigh I. M., Eady R. A., Heagerty A. H., Purkis P. E., Whitehead P. A., Burgeson R. E. Type VII collagen is a normal component of epidermal basement membrane, which shows altered expression in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 1988 May;90(5):639–642. doi: 10.1111/1523-1747.ep12560795. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases: role of the basement membrane. Warner-Lambert Parke-Davis Award lecture. Am J Pathol. 1984 Dec;117(3):339–348. [PMC free article] [PubMed] [Google Scholar]
- Nagle R. B., Böcker W., Davis J. R., Heid H. W., Kaufmann M., Lucas D. O., Jarasch E. D. Characterization of breast carcinomas by two monoclonal antibodies distinguishing myoepithelial from luminal epithelial cells. J Histochem Cytochem. 1986 Jul;34(7):869–881. doi: 10.1177/34.7.2423579. [DOI] [PubMed] [Google Scholar]
- Pitelka D. R., Hamamoto S. T., Taggart B. N. Basal lamina and tissue recognition in malignant mammary tumors. Cancer Res. 1980 May;40(5):1600–1611. [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Schaart G., Moesker O., Vooijs P. Tissue distribution of keratin 7 as monitored by a monoclonal antibody. Exp Cell Res. 1987 May;170(1):235–249. doi: 10.1016/0014-4827(87)90133-9. [DOI] [PubMed] [Google Scholar]
- Rao C. N., Margulies I. M., Tralka T. S., Terranova V. P., Madri J. A., Liotta L. A. Isolation of a subunit of laminin and its role in molecular structure and tumor cell attachment. J Biol Chem. 1982 Aug 25;257(16):9740–9744. [PubMed] [Google Scholar]
- Siegal G. P., Barsky S. H., Terranova V. P., Liotta L. A. Stages of neoplastic transformation of human breast tissue as monitored by dissolution of basement membrane components. An immunoperoxidase study. Invasion Metastasis. 1981;1(1):54–70. [PubMed] [Google Scholar]
- Warburton M. J., Head L. P., Rudland P. S. Redistribution of fibronectin and cytoskeletal proteins during the differentiation of rat mammary tumor cells in vitro. Exp Cell Res. 1981 Mar;132(1):57–66. doi: 10.1016/0014-4827(81)90082-3. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Mitchell D., Ormerod E. J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982 Jul;30(7):667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Willebrand D., Bosman F. T., de Goeij A. F. Patterns of basement membrane deposition in benign and malignant breast tumours. Histopathology. 1986 Dec;10(12):1231–1241. doi: 10.1111/j.1365-2559.1986.tb02567.x. [DOI] [PubMed] [Google Scholar]