Abstract
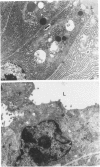
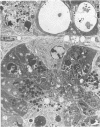
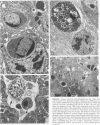
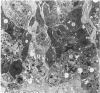
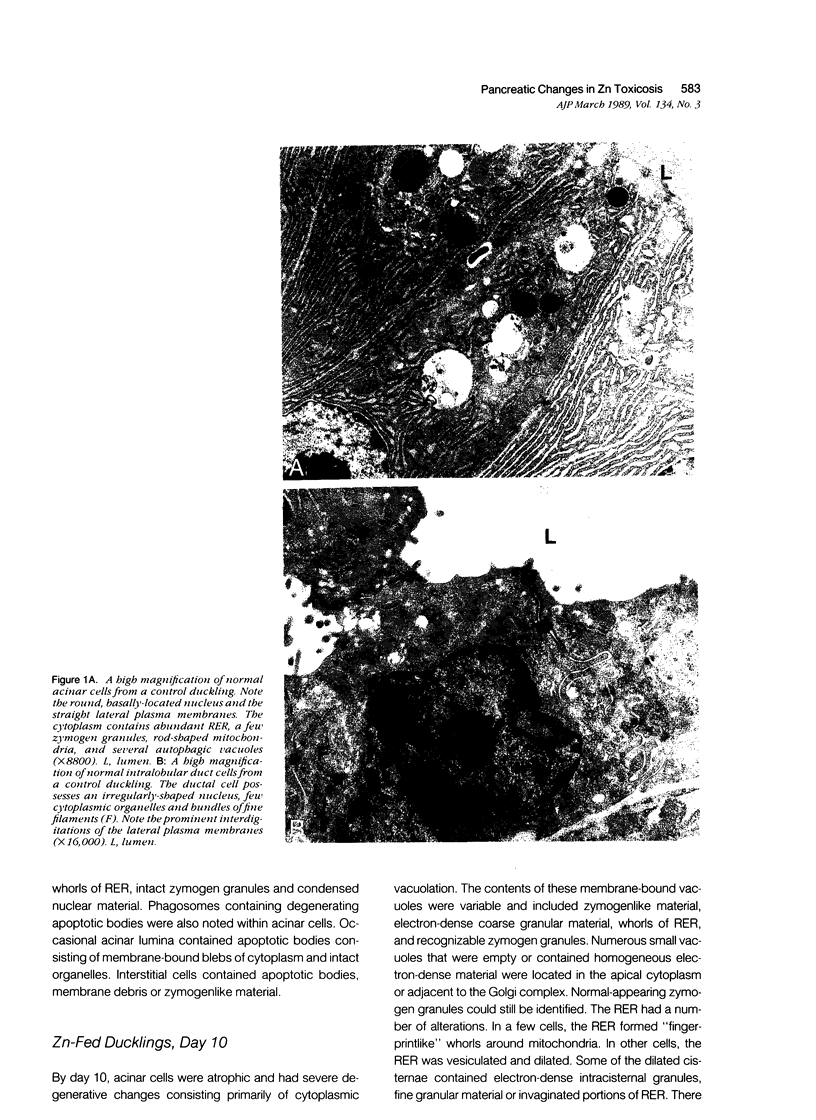
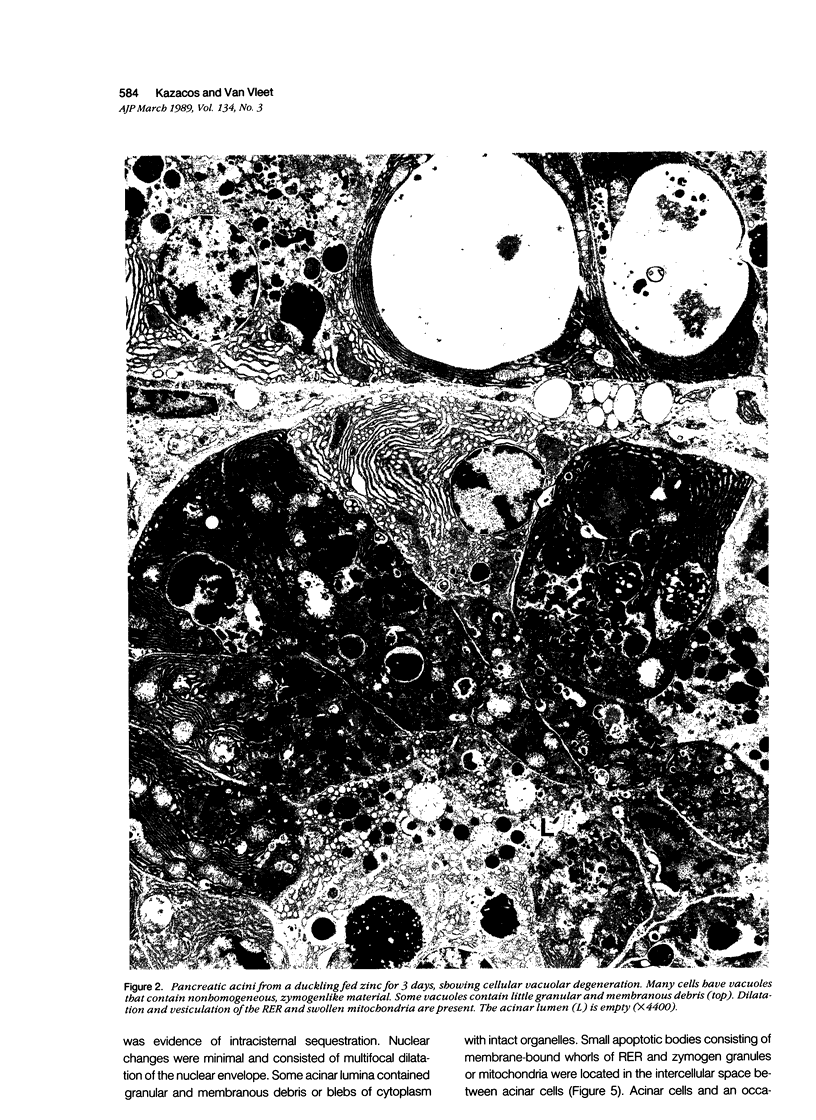
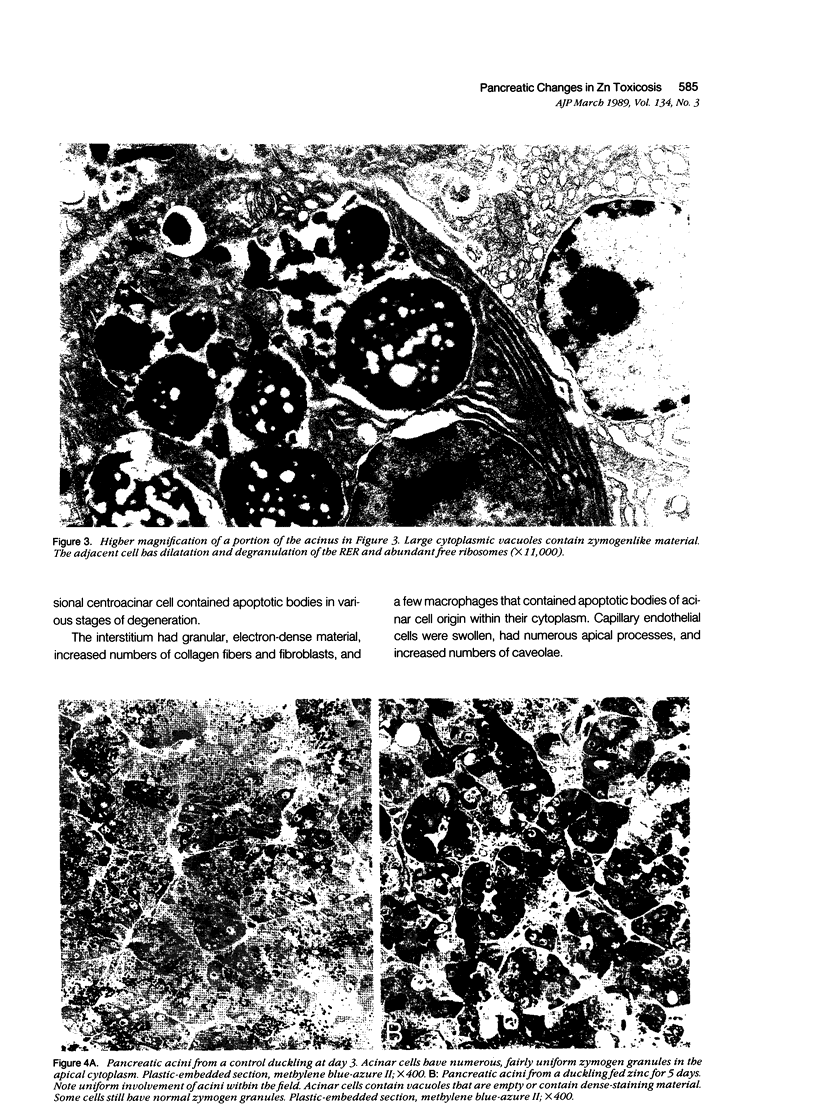
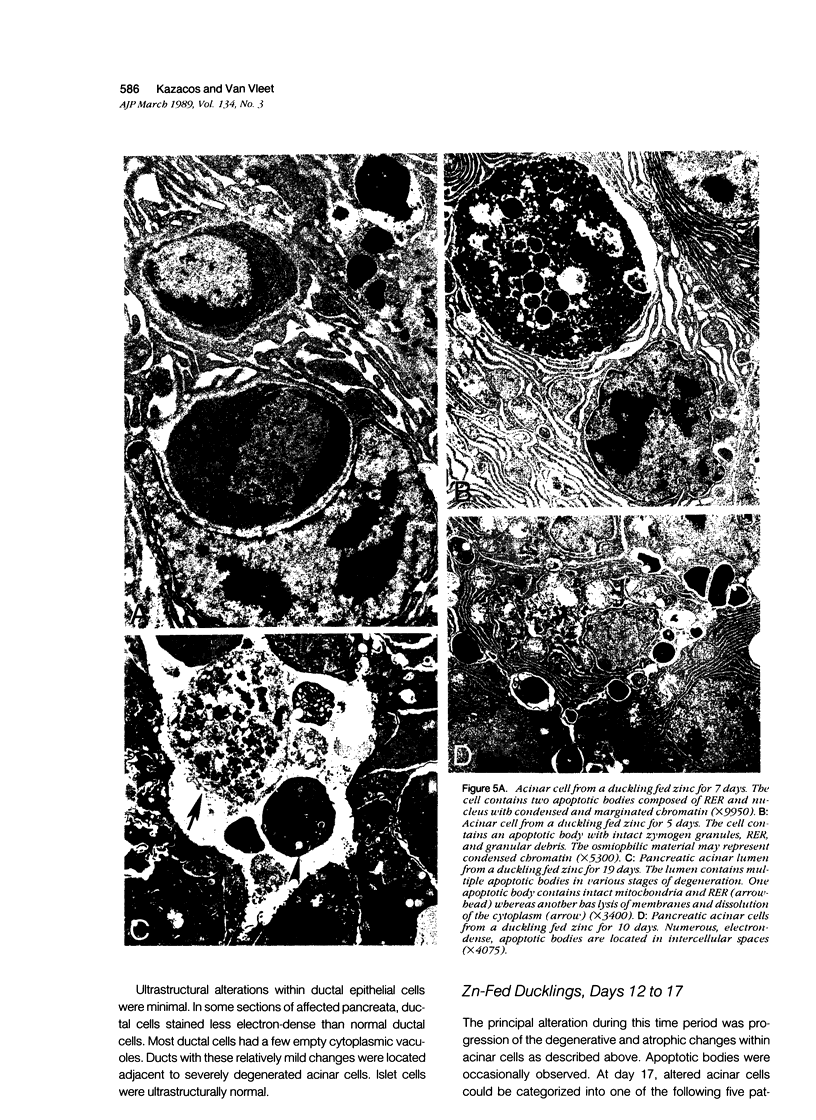
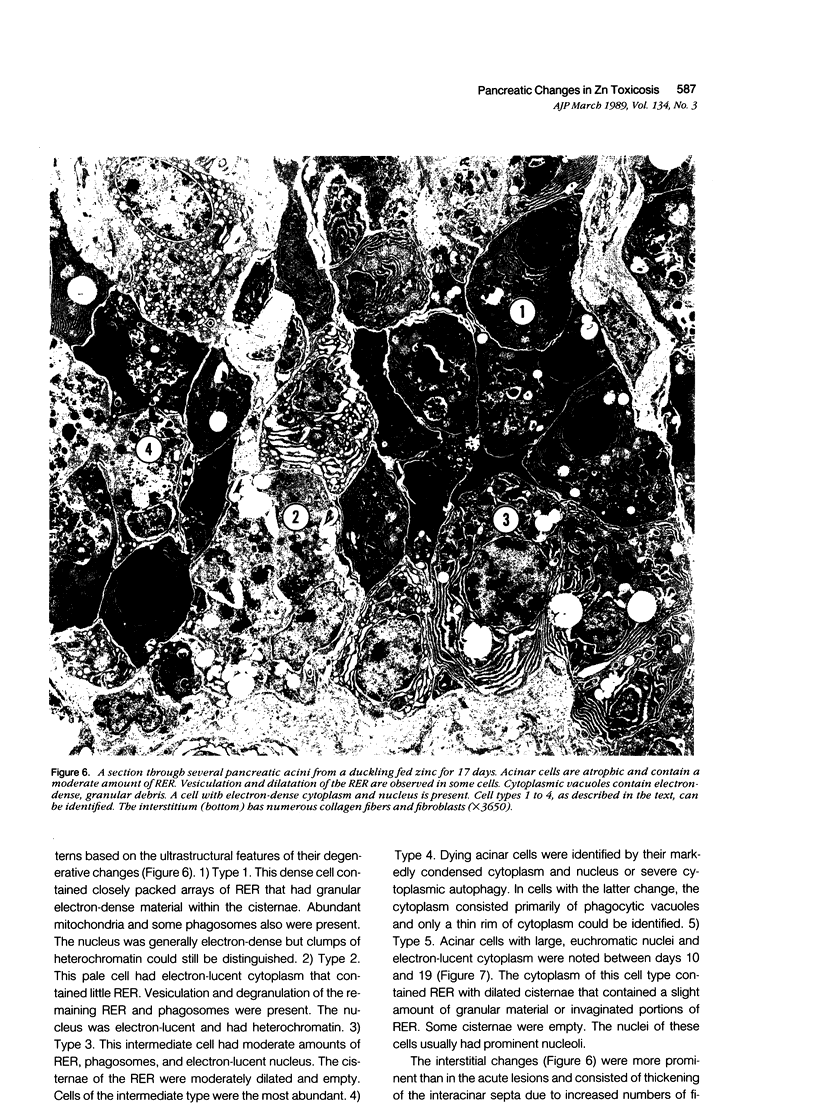
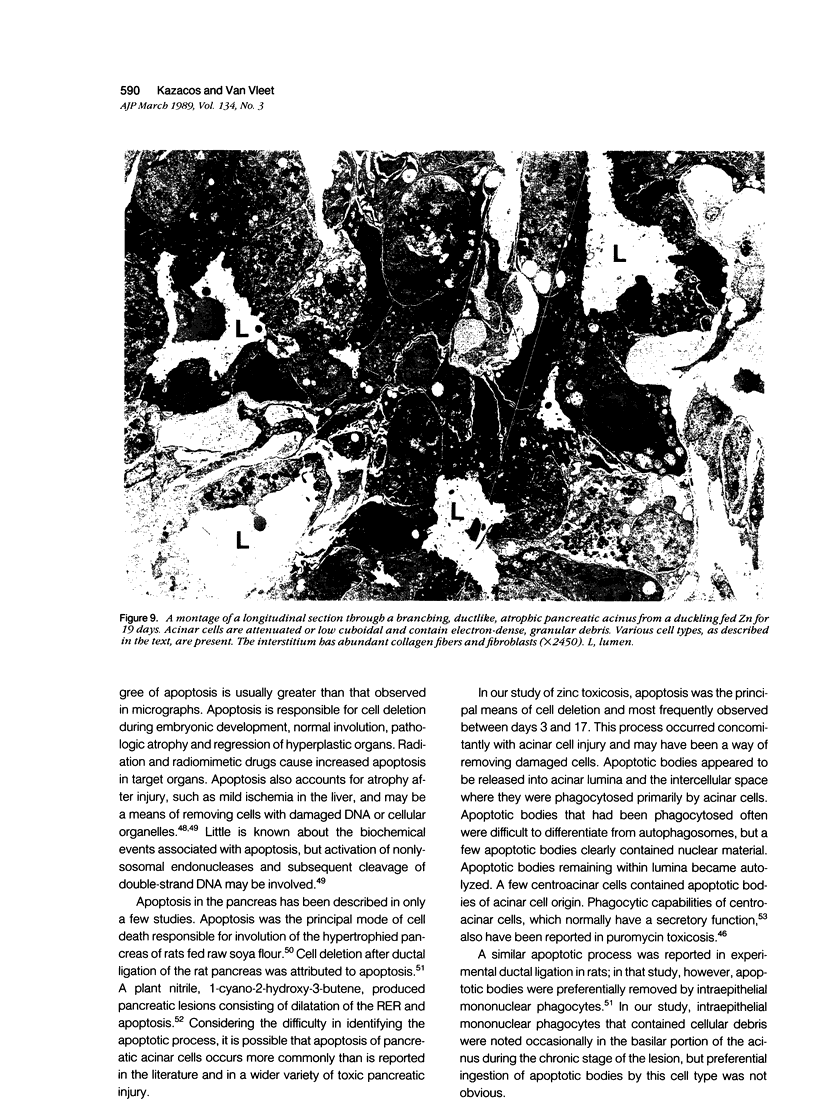
The sequential ultrastructural alterations of the pancreas in zinc toxicosis were examined in ducklings fed 2500 ppm Zn (as ZnSO4) for 56 days. From days 3 to 17, acinar cells had cytoplasmic vacuoles that contained electron-dense, zymogen-like material and increased autophagocytosis. Other changes were swollen mitochondria and dilatation, vesiculation, degranulation and intracisternal sequestration of rough endoplasmic reticulum. Apoptosis was the predominant form of cell deletion. By day 10, acinar cellular atrophy and interstitial fibrosis were noted. Islets appeared normal. After day 19, the pancreas consisted of ductlike structures embedded in fibrous connective tissue with a minimal inflammatory cell response. These ductlike structures were lined by attenuated to cuboidal, atrophic acinar cells. Many cells contained granular, electron-dense cytoplasmic debris that served as a marker of previous cell damage. This ultrastructural study provides support for a previously proposed theory that ductlike structures (tubular complexes) arise by atrophy and dedifferentiation of acinar cells.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. G., Masters H. G., Peet R. L., Mullins K. R., Lewis R. D., Skirrow S. Z., Fry J. Zinc toxicity in ruminants. J Comp Pathol. 1983 Jul;93(3):363–377. doi: 10.1016/0021-9975(83)90024-5. [DOI] [PubMed] [Google Scholar]
- Aughey E., Grant L., Furman B. L., Dryden W. F. The effects of oral zinc supplementation in the mouse. J Comp Pathol. 1977 Jan;87(1):1–14. doi: 10.1016/0021-9975(77)90074-3. [DOI] [PubMed] [Google Scholar]
- Blackburn W. R., Vinijchaikul K. The pancreas in kwashiorkor. An electron microscopic study. Lab Invest. 1969 Apr;20(4):305–318. [PubMed] [Google Scholar]
- Bockman D. E. Anastomosing tubular arrangement of dog exocrine pancreas. Cell Tissue Res. 1978 Jun 8;189(3):497–500. doi: 10.1007/BF00209135. [DOI] [PubMed] [Google Scholar]
- Bockman D. E. Anastomosing tubular arrangement of the exocrine pancreas. Am J Anat. 1976 Sep;147(1):113–118. doi: 10.1002/aja.1001470111. [DOI] [PubMed] [Google Scholar]
- Bockman D. E. Architecture of normal pancreas as revealed by retrograde injection. Cell Tissue Res. 1980;205(3):445–451. doi: 10.1007/BF00232285. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Black O., Jr, Mills L. R., Webster P. D. Origin of tubular complexes developing during induction of pancreatic adenocarcinoma by 7,12-dimethylbenz(a)anthracene. Am J Pathol. 1978 Mar;90(3):645–658. [PMC free article] [PubMed] [Google Scholar]
- Bockman D. E., Boydston W. R., Anderson M. C. Origin of tubular complexes in human chronic pancreatitis. Am J Surg. 1982 Aug;144(2):243–249. doi: 10.1016/0002-9610(82)90518-9. [DOI] [PubMed] [Google Scholar]
- Bockman D. E., Boydston W. R., Parsa I. Architecture of human pancreas: implications for early changes in pancreatic disease. Gastroenterology. 1983 Jul;85(1):55–61. [PubMed] [Google Scholar]
- Boquist L. Morphologic effects of ethionine on the pancreas of the Chinese hamster. A light and electron microscopic study of degenerative changes. Acta Pathol Microbiol Scand. 1969;76(1):91–105. doi: 10.1111/j.1699-0463.1969.tb03237.x. [DOI] [PubMed] [Google Scholar]
- Boquist L. The effect of excess methionine on the pancreas. A light and electron microscopic study in the Chinese hamster with particular reference to degenerative changes. Lab Invest. 1969 Aug;21(2):96–104. [PubMed] [Google Scholar]
- Bronson R. T., Strauss W., Wheeler W. Pancreatic ectasia in uremic macaques. Am J Pathol. 1982 Mar;106(3):342–347. [PMC free article] [PubMed] [Google Scholar]
- Case R. M. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. 1978 May;53(2):211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Dewar W. A., Wight P. A., Pearson R. A., Gentle M. J. Toxic effects of high concentrations of zinc oxide in the diet of the chick and laying hen. Br Poult Sci. 1983 Jul;24(3):397–404. doi: 10.1080/00071668308416754. [DOI] [PubMed] [Google Scholar]
- Etzel K. R., Cousins R. J. Hyperglycemic action of zinc in rats. J Nutr. 1983 Aug;113(8):1657–1663. doi: 10.1093/jn/113.8.1657. [DOI] [PubMed] [Google Scholar]
- Faintuch J., Faintuch J. J., Toledo M., Nazario G., Machado M. C., Raia A. A. Hyperamylasemia associated with zinc overdose during parenteral nutrition. JPEN J Parenter Enteral Nutr. 1978 Nov;2(5):640–645. doi: 10.1177/014860717800200504. [DOI] [PubMed] [Google Scholar]
- Fell B. F., Farmer L. J., Farquharson C., Bremner I., Graca D. S. Observations on the pancreas of cattle deficient in copper. J Comp Pathol. 1985 Oct;95(4):573–590. doi: 10.1016/0021-9975(85)90027-1. [DOI] [PubMed] [Google Scholar]
- Finkelbrand S., Coleman R., Silbermann M. The exocrine pancreas in triamcinolone-treated mice. A light and electron microscopy study. Acta Anat (Basel) 1978;102(4):348–357. doi: 10.1159/000145657. [DOI] [PubMed] [Google Scholar]
- Flaks B., Moore M. A., Flaks A. Ultrastructural analysis of pancreatic carcinogenesis. IV. Pseudoductular transformation of acini in the hamster pancreas during N-nitroso-bis(2-hydroxypropyl)amine carcinogenesis. Carcinogenesis. 1981;2(12):1241–1253. doi: 10.1093/carcin/2.12.1241. [DOI] [PubMed] [Google Scholar]
- Flaks B., Moore M. A., Flaks A. Ultrastructural analysis of pancreatic carcinogenesis. V. Changes in differentiation of acinar cells during chronic treatment with N-nitrosobis(2-hydroxypropyl)amine. Carcinogenesis. 1982;3(5):485–498. doi: 10.1093/carcin/3.5.485. [DOI] [PubMed] [Google Scholar]
- Flaks B., Moore M. A., Flaks A. Ultrastructural analysis of pancreatic carcinogenesis. VI. Early changes in hamster acinar cells induced by N-nitroso-bis(2-hydroxypropyl)amine. Carcinogenesis. 1982;3(9):1063–1070. doi: 10.1093/carcin/3.9.1063. [DOI] [PubMed] [Google Scholar]
- Helin H., Mero M., Markkula H., Helin M. Pancreatic acinar ultrastructure in human acute pancreatitis. Virchows Arch A Pathol Anat Histol. 1980;387(3):259–270. doi: 10.1007/BF00454829. [DOI] [PubMed] [Google Scholar]
- Inoue K., Fried G. M., Wiener I., Sakamoto T., Lilja P., Greeley G. H., Jr, Watson L. C., Thompson J. C. Effect of divalent cations on gastrointestinal hormone release and exocrine pancreatic secretion in dogs. Am J Physiol. 1985 Jan;248(1 Pt 1):G28–G34. doi: 10.1152/ajpgi.1985.248.1.G28. [DOI] [PubMed] [Google Scholar]
- Koike H., Steer M. L., Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982 Apr;242(4):G297–G307. doi: 10.1152/ajpgi.1982.242.4.G297. [DOI] [PubMed] [Google Scholar]
- LAZARUS S. S., VOLK B. W. ELECTRON MICROSCOPY AND HISTOCHEMISTRY OF RABBIT PANCREAS IN PROTEIN MALNUTRITION (EXPERIMENTAL KWASHIORKOR). Am J Pathol. 1964 Jan;44:95–111. [PMC free article] [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Leong A. S., Slavotinek A. H., Deakin E. J., Nance S. H., Elmslie R. G. The pathology of experimental chronic fibrosing pancreatitis--light microscopic and ultrastructural observations. Pathology. 1982 Oct;14(4):363–368. doi: 10.3109/00313028209092112. [DOI] [PubMed] [Google Scholar]
- Logsdon C. D., Moessner J., Williams J. A., Goldfine I. D. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pancreatic acinar AR42J cells. J Cell Biol. 1985 Apr;100(4):1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi B., Estes L. W., Longnecker D. S. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by DL-ethionine fed with a choline-deficient diet. Am J Pathol. 1975 Jun;79(3):465–480. [PMC free article] [PubMed] [Google Scholar]
- Lombardi B. Influence of dietary factors on the pancreatotoxicity of ethionine. Am J Pathol. 1976 Sep;84(3):633–648. [PMC free article] [PubMed] [Google Scholar]
- Longnecker D. S. Pathology and pathogenesis of diseases of the pancreas. Am J Pathol. 1982 Apr;107(1):99–121. [PMC free article] [PubMed] [Google Scholar]
- Longnecker D. S., Shinozuka H., Farber E. Molecular pathology of in-vivo inhibition of protein synthesis. Electron microscopy of rat pancreatic acinar cells in puromycin-induced necrosis. Am J Pathol. 1968 Apr;52(4):891–915. [PMC free article] [PubMed] [Google Scholar]
- Masoero G., Wormsley K. G. Functional interrelationships of exocrine pancreas and endocrine glands in health and disease. Mt Sinai J Med. 1980 May-Jun;47(3):261–272. [PubMed] [Google Scholar]
- Nasset E. S. Role of the digestive system in protein metabolism. Fed Proc. 1965 Jul-Aug;24(4):953–958. [PubMed] [Google Scholar]
- Nevalainen T. J., Janigan D. T. Degeneration of mouse pancreatic acinar cells during fasting. Virchows Arch B Cell Pathol. 1974 Mar 19;15(2):107–118. doi: 10.1007/BF02889329. [DOI] [PubMed] [Google Scholar]
- PORTA E. A., STEIN A. A., PATTERSON P. ULTRASTRUCTURAL CHANGES OF THE PANCREAS AND LIVER IN CYSTIC FIBROSIS. Am J Clin Pathol. 1964 Nov;42:451–465. doi: 10.1093/ajcp/42.5.451. [DOI] [PubMed] [Google Scholar]
- Pound A. W., Walker N. I. Involution of the pancreas after ligation of the pancreatic ducts. I: a histological study. Br J Exp Pathol. 1981 Dec;62(6):547–558. [PMC free article] [PubMed] [Google Scholar]
- Pour P. M. Mechanism of pseudoductular (tubular) formation during pancreatic carcinogenesis in the hamster model. An electron-microscopic and immunohistochemical study. Am J Pathol. 1988 Feb;130(2):335–344. [PMC free article] [PubMed] [Google Scholar]
- Racela A. S., Jr, Grady H. J., Higginson J., Svoboda D. J. Protein deficiency in rhesus monkeys. Am J Pathol. 1966 Sep;49(3):419–443. [PMC free article] [PubMed] [Google Scholar]
- Rebar A. H., Van Vleet J. F. Ultrastructural changes in the pancreata of selenium-vitamin E-deficient chicks. Vet Pathol. 1977 Nov;14(6):629–642. doi: 10.1177/030098587701400609. [DOI] [PubMed] [Google Scholar]
- Resau J. H., Marzella L., Trump B. F., Jones R. T. Degradation of zymogen granules by lysosomes in cultured pancreatic explants. Am J Pathol. 1984 May;115(2):139–150. [PMC free article] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Smith P. A., Sunter J. P., Case R. M. Progressive atrophy of pancreatic acinar tissue in rats fed a copper-deficient diet supplemented with D-penicillamine or triethylene tetramine: morphological and physiological studies. Digestion. 1982;23(1):16–30. doi: 10.1159/000198706. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Meldolesi J., Figarella C. Pancreatitis. The role of lysosomes. Dig Dis Sci. 1984 Oct;29(10):934–938. doi: 10.1007/BF01312483. [DOI] [PubMed] [Google Scholar]
- Sugarman B. Zinc and infection. Rev Infect Dis. 1983 Jan-Feb;5(1):137–147. doi: 10.1093/clinids/5.1.137. [DOI] [PubMed] [Google Scholar]
- Svoboda D., Grady H., Higginson J. The effects of chronic protein deficiency in rats. II. Biochemical and ultrastructural changes. Lab Invest. 1966 Apr;15(4):731–749. [PubMed] [Google Scholar]
- Van Vleet J. F., Boon G. D., Ferrans V. J. Induction of lesions of selenium-vitamin E deficiency in ducklings fed silver, copper, cobalt, tellurium, cadmium, or zinc: protection by selenium or vitamin E supplements. Am J Vet Res. 1981 Jul;42(7):1206–1217. [PubMed] [Google Scholar]
- WEISBLUM B., HERMAN L., FITZGERALD P. J. Changes in pancreatic acinar cells during protein deprivation. J Cell Biol. 1962 Feb;12:313–327. doi: 10.1083/jcb.12.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N. I. Ultrastructure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol. 1987 Mar;126(3):439–451. [PMC free article] [PubMed] [Google Scholar]
- Wallig M. A., Gould D. H., Fettman M. J. Selective pancreato-toxicity in the rat induced by the naturally occurring plant nitrile 1-cyano-2-hydroxy-3-butene. Food Chem Toxicol. 1988 Feb;26(2):137–147. doi: 10.1016/0278-6915(88)90110-x. [DOI] [PubMed] [Google Scholar]
- Watanabe O., Baccino F. M., Steer M. L., Meldolesi J. Supramaximal caerulein stimulation and ultrastructure of rat pancreatic acinar cell: early morphological changes during development of experimental pancreatitis. Am J Physiol. 1984 Apr;246(4 Pt 1):G457–G467. doi: 10.1152/ajpgi.1984.246.4.G457. [DOI] [PubMed] [Google Scholar]
- Willemer S., Elsässer H. P., Kern H. F., Adler G. Tubular complexes in cerulein- and oleic acid-induced pancreatitis in rats: glycoconjugate pattern, immunocytochemical, and ultrastructural findings. Pancreas. 1987;2(6):669–675. doi: 10.1097/00006676-198711000-00008. [DOI] [PubMed] [Google Scholar]
- ZELANDER T., EKHOLM R., EDLUND Y. THE ULTRASTRUCTURE OF THE RAT EXOCRINE PANCREAS AFTER EXPERIMENTALLY OCCLUDED OUTFLOW. J Ultrastruct Res. 1964 Feb;10:89–102. doi: 10.1016/s0022-5320(64)90023-1. [DOI] [PubMed] [Google Scholar]
- Zeligs J. D., Janoff A., Dumont A. E. The course and nature of acinar cell death following pancreatic ligation in the guinea pig. Am J Pathol. 1975 Aug;80(2):203–226. [PMC free article] [PubMed] [Google Scholar]