Abstract
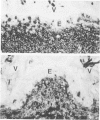
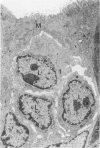
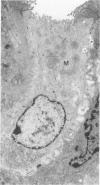
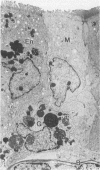
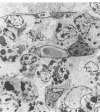
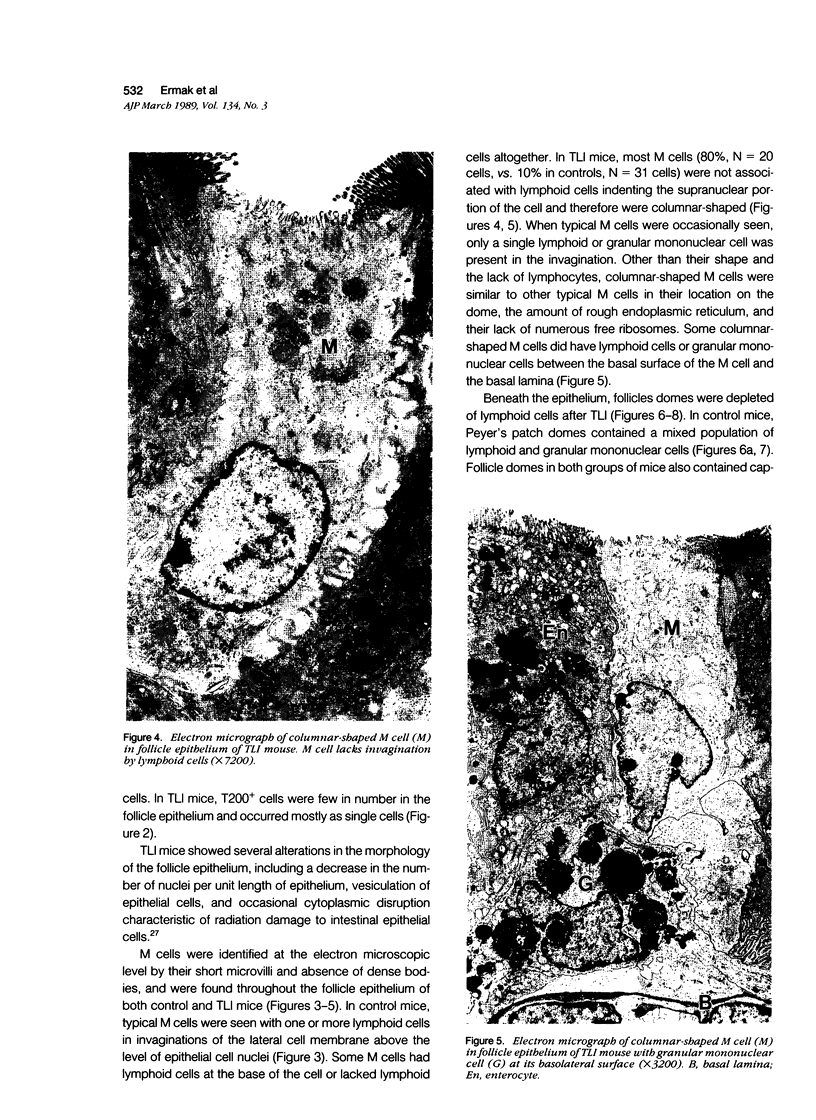
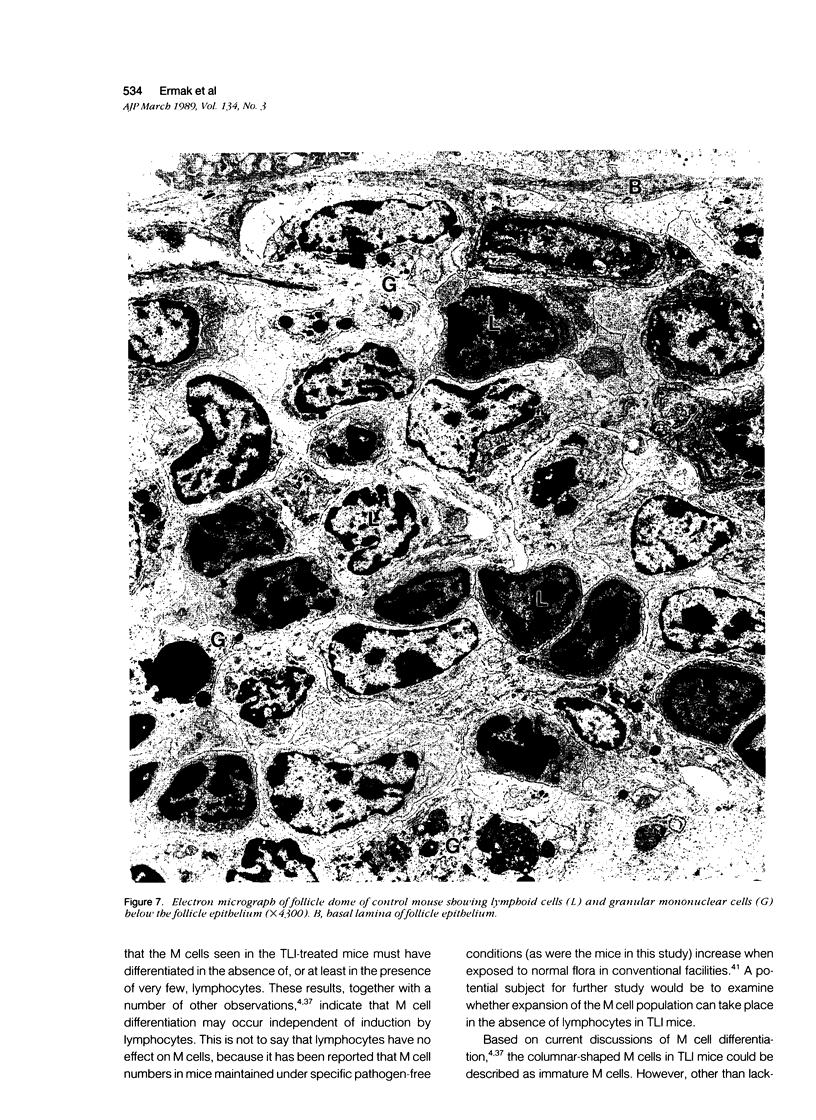
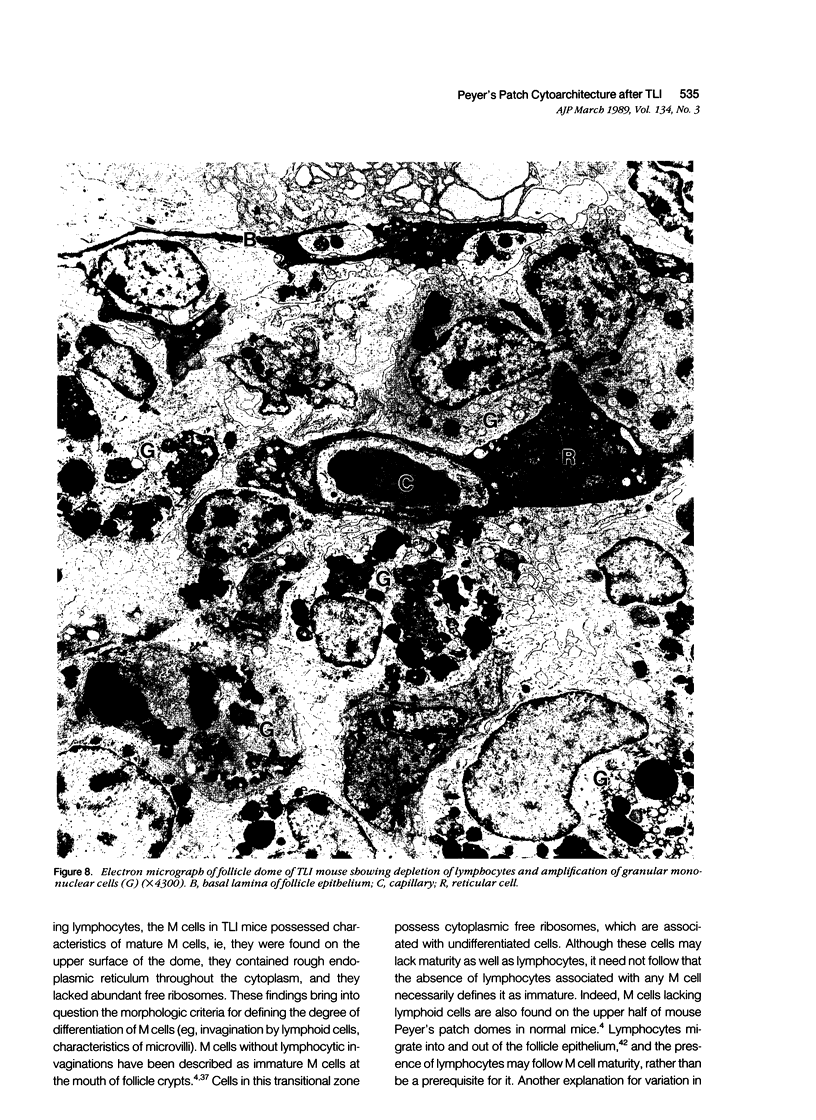
The cytoarchitecture of Peyer's patches that were depleted of their lymphocytes by total lymphoid irradiation (TLI) was examined with particular attention to the effects on M cells in the follicle epithelium and on mononuclear cells in follicle domes underlying the epithelium. Five-month-old, specific pathogen-free Balb/c mice were irradiated with 200-250 rad/day, five times a week to a total dose of 3400-4250, and their Peyer's patches were either fixed for electron microscopy or frozen for immunohistochemistry 1-4 days after completion of irradiation. Control mice were examined at the same time intervals. Follicle domes of TLI mice had approximately one fourth the epithelial surface area of domes of control mice. Within the epithelium, lymphoid cells were virtually depleted after TLI, and yet the epithelium contained M cells. In control mice, most M cells were accompanied by lymphoid cells in invaginations of the apical-lateral cell membrane. In TLI mice, most M cells did not have such apical-lateral invaginations and were columnar shaped. Other than lacking lymphocytes, these cells appeared to be mature M cells. Some M cells did have lymphoid cells or granular mononuclear cells below their basal membranes, adjacent to the basal lamina. Below the epithelium, the proportion of granular mononuclear cells was greatly increased following TLI. The retention of M cells and the increase in proportion of granular mononuclear cells in follicle domes are consistent with selective depletion of lymphocytes following TLI. Persistence of M cells without lymphocytic invaginations after TLI suggests that M cells can differentiate in the absence of, or at least in the presence of very few, lymphocytes, and that invagination by lymphocytes is not necessary to maintain mature M cell morphology.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Ito T. Fine structure of the dome in Peyer's patches of mice. Arch Histol Jpn. 1978 Jun;41(3):195–204. doi: 10.1679/aohc1950.41.195. [DOI] [PubMed] [Google Scholar]
- Bhalla D. K., Murakami T., Owen R. L. Microcirculation of intestinal lymphoid follicles in rat Peyer's patches. Gastroenterology. 1981 Sep;81(3):481–491. [PubMed] [Google Scholar]
- Bhalla D. K., Owen R. L. Cell renewal and migration in lymphoid follicles of Peyer's patches and cecum--an autoradiographic study in mice. Gastroenterology. 1982 Feb;82(2):232–242. [PubMed] [Google Scholar]
- Bhalla D. K., Owen R. L. Migration of B and T lymphocytes to M cells in Peyer's patch follicle epithelium: an autoradiographic and immunocytochemical study in mice. Cell Immunol. 1983 Oct 1;81(1):105–117. doi: 10.1016/0008-8749(83)90216-2. [DOI] [PubMed] [Google Scholar]
- Blythman H. E., Waksman B. H. Effect of irradiation and appendicostomy on appendix structure and responses of appendix cells to mitogens. J Immunol. 1973 Jul;111(1):171–182. [PubMed] [Google Scholar]
- Bye W. A., Allan C. H., Trier J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984 May;86(5 Pt 1):789–801. [PubMed] [Google Scholar]
- Cheng H., Leblond C. P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974 Dec;141(4):537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Differential distribution of lymphocytes and accessory cells in mouse Peyer's patches. Anat Rec. 1986 Jun;215(2):144–152. doi: 10.1002/ar.1092150208. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Owen R. L. Phenotype and distribution of T lymphocytes in Peyer's patches of athymic mice. Histochemistry. 1987;87(4):321–325. doi: 10.1007/BF00492585. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J., Owen R. L., Heyworth M. F. Modulation of lymphocyte subsets in Peyer's patches of mice treated with monoclonal antibody against helper T-cells. J Histochem Cytochem. 1988 Apr;36(4):417–423. doi: 10.1177/36.4.2964470. [DOI] [PubMed] [Google Scholar]
- Ermak T. H., Steger H. J., Owen R. L., Strober S. Depletion and repopulation of lymphocytes in Peyer's patches of mice after total lymphoid irradiation. Lab Invest. 1988 Nov;59(5):591–597. [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer's patch in Escherichia coli diarrhea in the rabbit. J Clin Invest. 1983 Jan;71(1):1–8. doi: 10.1172/JCI110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lause D. B., Bockman D. E. Heterogeneity, position, and functional capability of the macrophages in Peyer's patches. Cell Tissue Res. 1981;218(3):557–566. doi: 10.1007/BF00210115. [DOI] [PubMed] [Google Scholar]
- LeFevre M. E., Hammer R., Joel D. D. Macrophages of the mammalian small intestine: a review. J Reticuloendothel Soc. 1979 Nov;26(5):553–573. [PubMed] [Google Scholar]
- Madara J. L., Bye W. A., Trier J. S. Structural features of and cholesterol distribution in M-cell membranes in guinea pig, rat, and mouse Peyer's patches. Gastroenterology. 1984 Nov;87(5):1091–1103. [PubMed] [Google Scholar]
- Marcial M. A., Madara J. L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986 Mar;90(3):583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- Neutra M. R., Phillips T. L., Mayer E. L., Fishkind D. J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer's patch. Cell Tissue Res. 1987 Mar;247(3):537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- Oseroff A., Okada S., Strober S. Natural suppressor (NS) cells found in the spleen of neonatal mice and adult mice given total lymphoid irradiation (TLI) express the null surface phenotype. J Immunol. 1984 Jan;132(1):101–110. [PubMed] [Google Scholar]
- Owen R. L., Allen C. L., Stevens D. P. Phagocytosis of Giardia muris by macrophages in Peyer's patch epithelium in mice. Infect Immun. 1981 Aug;33(2):591–601. doi: 10.1128/iai.33.2.591-601.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Apple R. T., Bhalla D. K. Morphometric and cytochemical analysis of lysosomes in rat Peyer's patch follicle epithelium: their reduction in volume fraction and acid phosphatase content in M cells compared to adjacent enterocytes. Anat Rec. 1986 Dec;216(4):521–527. doi: 10.1002/ar.1092160409. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Bhalla D. K. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer's patch M cells. Am J Anat. 1983 Oct;168(2):199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pappo J., Owen R. L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988 Nov;95(5):1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- Rowiński J., Lamprecht J., Siciński P. Non-random distribution of intraepithelial lymphoid cells in follicle-associated epithelium of Peyer's patches in mice. J Anat. 1984 Aug;139(Pt 1):21–32. [PMC free article] [PubMed] [Google Scholar]
- Siciński P., Rowiński J., Warchoł J. B., Bem W. Morphometric evidence against lymphocyte-induced differentiation of M cells from absorptive cells in mouse Peyer's patches. Gastroenterology. 1986 Mar;90(3):609–616. doi: 10.1016/0016-5085(86)91114-5. [DOI] [PubMed] [Google Scholar]
- Slavin S., Strober S., Fuks Z., Kaplan H. S. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977 Jul 1;146(1):34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., James P. S., Tivey D. R. M cell numbers increase after transfer of SPF mice to a normal animal house environment. Am J Pathol. 1987 Sep;128(3):385–389. [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Jarvis L. G., King I. S. Cell proliferation in follicle-associated epithelium of mouse Peyer's patch. Am J Anat. 1980 Oct;159(2):157–166. doi: 10.1002/aja.1001590204. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Peacock M. A. "M" cell distribution in follicle-associated epithelium of mouse Peyer's patch. Am J Anat. 1980 Oct;159(2):167–175. doi: 10.1002/aja.1001590205. [DOI] [PubMed] [Google Scholar]
- Smith M. W. Selective expression of brush border hydrolases by mouse Peyer's patch and jejunal villus enterocytes. J Cell Physiol. 1985 Aug;124(2):219–225. doi: 10.1002/jcp.1041240208. [DOI] [PubMed] [Google Scholar]
- Sobhon P. The light and the electron microscopic studies of Peyer's patches in non germ-free adult mice. J Morphol. 1971 Dec;135(4):457–481. doi: 10.1002/jmor.1051350404. [DOI] [PubMed] [Google Scholar]
- Strober S. 'Managing' the immune system with total lymphoid irradiation. Hosp Pract (Off Ed) 1981 Jun;16(6):77–89. doi: 10.1080/21548331.1981.11946785. [DOI] [PubMed] [Google Scholar]
- Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu Rev Immunol. 1984;2:219–237. doi: 10.1146/annurev.iy.02.040184.001251. [DOI] [PubMed] [Google Scholar]
- Trier J. S., Browning T. H. Morphologic response of the mucosa of human small intestine to x-ray exposure. J Clin Invest. 1966 Feb;45(2):194–204. doi: 10.1172/JCI105332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J. L., Kauffman R. S., Finberg R., Dambrauskas R., Fields B. N., Trier J. S. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983 Aug;85(2):291–300. [PubMed] [Google Scholar]