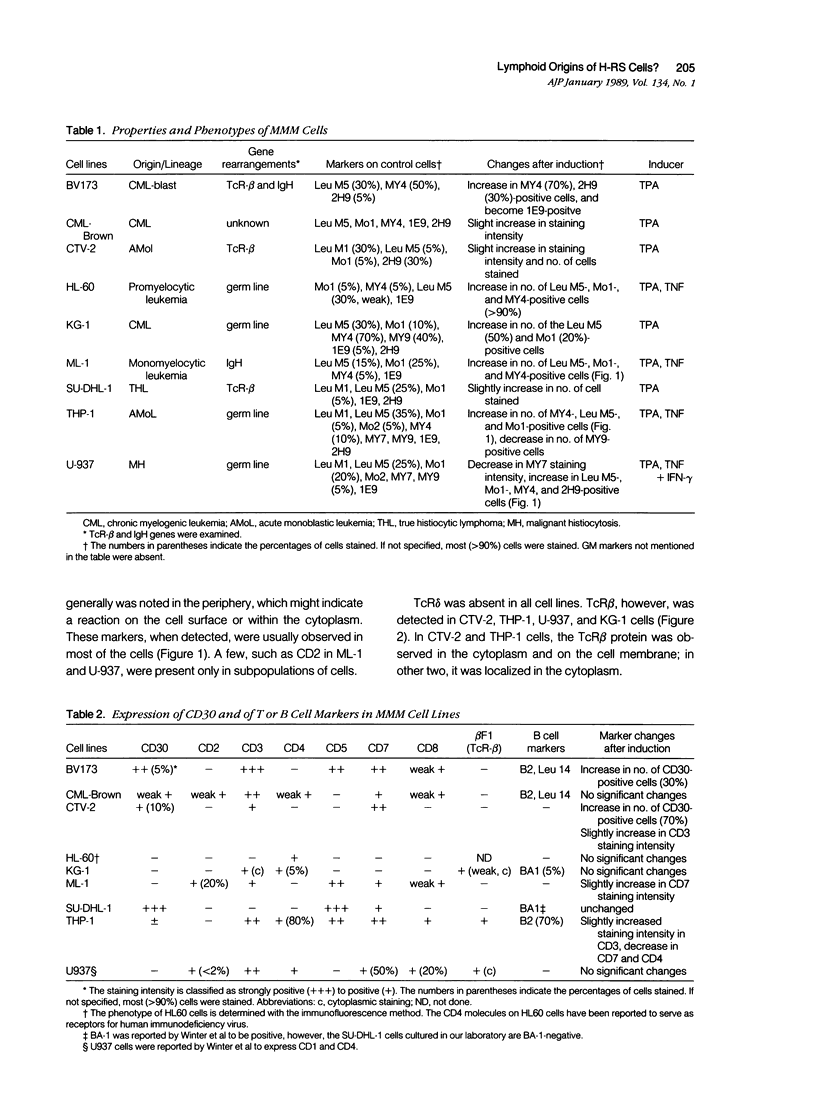
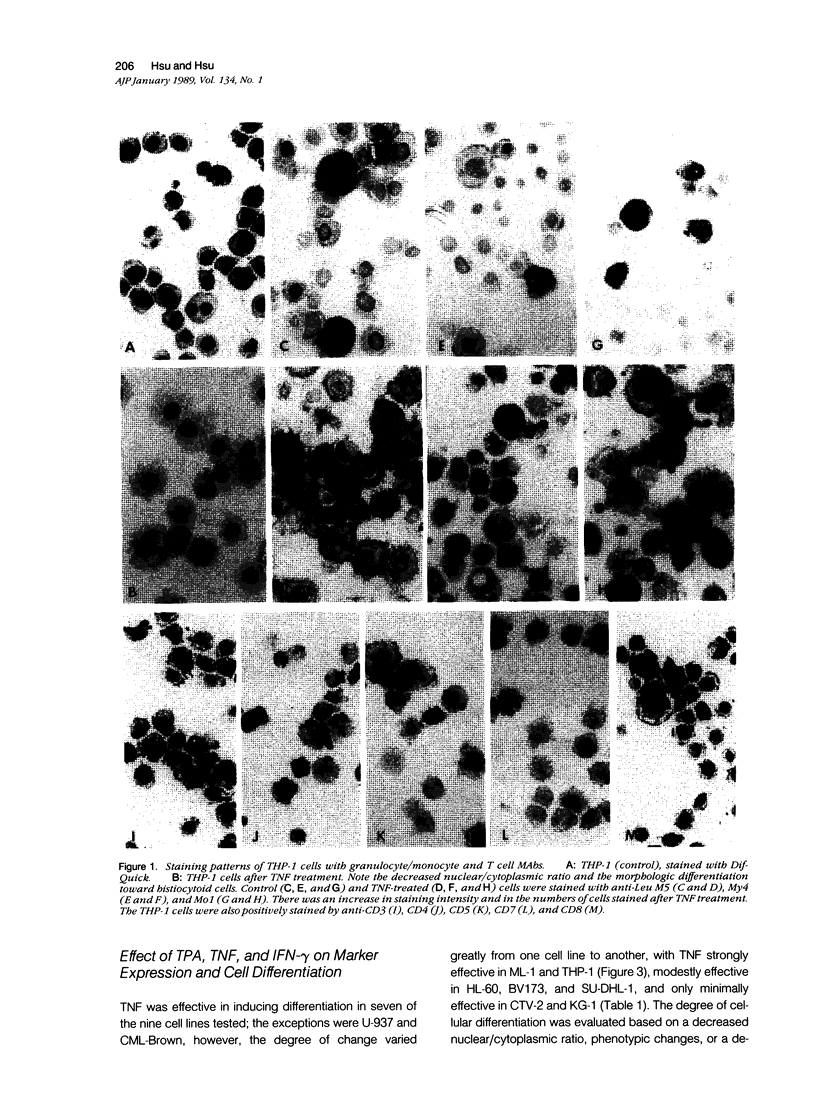
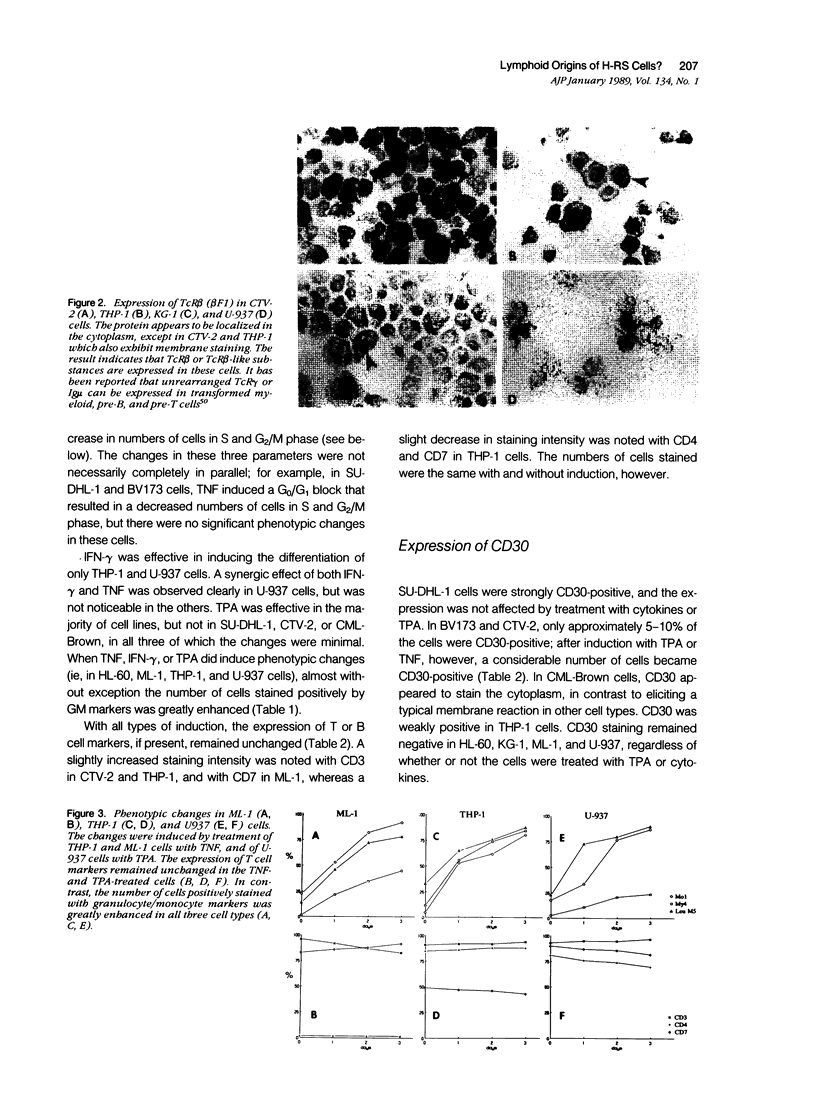
Abstract
Most Hodgkin's mononuclear cells and Reed-Sternberg (H-RS) cells are characterized by the expression of the antigen CD30, but not of T or B cell markers. A few H-RS cells, however, may express a limited number of T or B cell markers. Whether this expression is sufficient to allow the conclusion that H-RS cells are derived from T and/or B cells has been debated vigorously. The present study examined whether CD30 and aberrant T and B cell markers are expressed in cell lines that are well documented as being derived from the granulocyte/monocyte/histiocyte lineage. These cells included HL-60, KG-1, ML-1, THP-1, and U-937. Four other cell lines derived from patients with leukemias/lymphomas of monocytic or granulocytic origins also were studied. These cells included BV173, CML-Brown, CTV-2, and SU-DHL-1. If aberrant expression is detected, by analogy one may expect that rare T or B cell marker expression may occur in H-RS cells, because abundant evidence has indicated that H-RS cells may be related to cells in histiocyte lineage. In all nine of the cell lines studied, it was confirmed that numerous monocyte/granulocyte markers were expressed. The marker expression was enhanced after cells were induced to differentiate with phorbol ester (TPA) and tumor necrosis factor (TNF). It was noted that several T and B cell markers also were expressed by these cells. Unlike the expression of monocyte/granulocyte markers, the expression of T or B cell markers was not affected, or only minimally affected, by treatment of the cells with TPA or TNF. Five of the cell lines (BV173, CML-Brown, CTV-2, SU-DHL-1, and THP-1) were shown to be CD30-positive. In CTV-2 and BV173, the expression of CD30 was greatly increased after induction with phorbol ester or TNF. Based on these studies, the following conclusions were reached: 1) The expression of aberrant B or T cell markers is not an uncommon finding in granulocyte/monocyte/histiocyte-related neoplastic cells. 2) The expression of granulocyte/monocyte markers in these cells is related to the state of cell differentiation, whereas the expression of T or B cell markers is not. 3) CD30 is not necessarily a proliferation-related antigen, and its expression is not a sole property of T or B cells, but can be present in granulocyte/monocyte/histiocyte-related cells.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen R., Gadd S., Brugger W., Löhr G. W., Atkins R. C. Activation of human monocyte-derived macrophages cultured on Teflon: response to interferon-gamma during terminal maturation in vitro. Immunobiology. 1988 May;177(2):186–198. doi: 10.1016/s0171-2985(88)80038-x. [DOI] [PubMed] [Google Scholar]
- Andreesen R., Osterholz J., Löhr G. W., Bross K. J. A Hodgkin cell-specific antigen is expressed on a subset of auto- and alloactivated T (helper) lymphoblasts. Blood. 1984 Jun;63(6):1299–1302. [PubMed] [Google Scholar]
- Band H., Hochstenbach F., McLean J., Hata S., Krangel M. S., Brenner M. B. Immunochemical proof that a novel rearranging gene encodes the T cell receptor delta subunit. Science. 1987 Oct 30;238(4827):682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Scheft H., Warnke R. A., Jones N., Strominger J. L. Characterization and expression of the human alpha beta T cell receptor by using a framework monoclonal antibody. J Immunol. 1987 Mar 1;138(5):1502–1509. [PubMed] [Google Scholar]
- Burrichter H., Heit W., Schaadt M., Kirchner H., Diehl V. Production of colony-stimulating factors by Hodgkin cell lines. Int J Cancer. 1983 Mar 15;31(3):269–274. doi: 10.1002/ijc.2910310303. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L. C., Greaves M. F. Acute leukaemia with mixed lymphoid and myeloid phenotype. Br J Haematol. 1984 Sep;58(1):203–205. doi: 10.1111/j.1365-2141.1984.tb06075.x. [DOI] [PubMed] [Google Scholar]
- Chen P., Chiu C., Chiou T., Maeda S., Chiang H., Tzeng C., Sugiyama T., Chiang B. N. Establishment and characterization of a human monocytoid leukemia cell line, CTV-1. Gan. 1984 Aug;75(8):660–664. [PubMed] [Google Scholar]
- Cook W. D., Balaton A. M. T-cell receptor and immunoglobulin genes are rearranged together in Abelson virus-transformed pre-B and pre-T cells. Mol Cell Biol. 1987 Jan;7(1):266–272. doi: 10.1128/mcb.7.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossman J., Neckers L. M., Hsu S., Longo D., Jaffe E. S. Low-grade lymphomas. Expression of developmentally regulated B-cell antigens. Am J Pathol. 1984 Apr;115(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Drexler H. G., Gaedicke G., Lok M. S., Diehl V., Minowada J. Hodgkin's disease derived cell lines HDLM-2 and L-428: comparison of morphology, immunological and isoenzyme profiles. Leuk Res. 1986;10(5):487–500. doi: 10.1016/0145-2126(86)90084-6. [DOI] [PubMed] [Google Scholar]
- Epstein A. L., Levy R., Kim H., Henle W., Henle G., Kaplan H. S. Biology of the human malignant lymphomas. IV. Functional characterization of ten diffuse histiocytic lymphoma cell lines. Cancer. 1978 Nov;42(5):2379–2391. doi: 10.1002/1097-0142(197811)42:5<2379::aid-cncr2820420539>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Fischer P., Nacheva E., Mason D. Y., Sherrington P. D., Hoyle C., Hayhoe F. G., Karpas A. A Ki-1 (CD30)-positive human cell line (Karpas 299) established from a high-grade non-Hodgkin's lymphoma, showing a 2;5 translocation and rearrangement of the T-cell receptor beta-chain gene. Blood. 1988 Jul;72(1):234–240. [PubMed] [Google Scholar]
- Fisher R. I., Bates S. E., Bostick-Bruton F., Tuteja N., Diehl V. Neoplastic cells obtained from Hodgkin's disease function as accessory cells for mitogen-induced human T cell proliferative responses. J Immunol. 1984 May;132(5):2672–2677. [PubMed] [Google Scholar]
- Fisher R. I., Bostick-Bruton F., Sauder D. N., Scala G., Diehl V. Neoplastic cells obtained from Hodgkin's disease are potent stimulators of human primary mixed lymphocyte cultures. J Immunol. 1983 Jun;130(6):2666–2670. [PubMed] [Google Scholar]
- Greaves M. F., Chan L. C., Furley A. J., Watt S. M., Molgaard H. V. Lineage promiscuity in hemopoietic differentiation and leukemia. Blood. 1986 Jan;67(1):1–11. [PubMed] [Google Scholar]
- Hecht T. T., Longo D. L., Cossman J., Bolen J. B., Hsu S. M., Israel M., Fisher R. I. Production and characterization of a monoclonal antibody that binds Reed-Sternberg cells. J Immunol. 1985 Jun;134(6):4231–4236. [PubMed] [Google Scholar]
- Ho Y. S., Subhendu C., Hsu S. M. Induction of differentiation of African Burkitt's lymphoma cells by phorbol ester: possible relation with early B cells. Cancer Invest. 1987;5(2):101–107. doi: 10.3109/07357908709018463. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Cossman J., Jaffe E. S. Lymphocyte subsets in normal human lymphoid tissues. Am J Clin Pathol. 1983 Jul;80(1):21–30. doi: 10.1093/ajcp/80.1.21. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Ho Y. S., Li P. J., Monheit J., Ree H. J., Sheibani K., Winberg C. D. L&H variants of Reed-Sternberg cells express sialylated Leu M1 antigen. Am J Pathol. 1986 Feb;122(2):199–203. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L., Lo S. S., Wu K. K. Expression of prostaglandin H synthase (cyclooxygenase) in Hodgkin's mononuclear and Reed-Sternberg cells. Functional resemblance between H-RS cells and histiocytes or interdigitating reticulum cells. Am J Pathol. 1988 Oct;133(1):5–12. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Hsu P. L. Phenotypes and phorbol ester-induced differentiation of human histiocytic lymphoma cell lines (U-937 and SU-DHL-1) and Reed-Sternberg cells. Am J Pathol. 1986 Feb;122(2):223–230. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Leu M1 and peanut agglutinin stain the neoplastic cells of Hodgkin's disease. Am J Clin Pathol. 1984 Jul;82(1):29–32. doi: 10.1093/ajcp/82.1.29. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of B-lymphocytes. 1. Identification with monoclonal antibodies in normal lymphoid tissues. Am J Pathol. 1984 Mar;114(3):387–395. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Jaffe E. S. Phenotypic expression of T lymphocytes in thymus and peripheral lymphoid tissues. Am J Pathol. 1985 Oct;121(1):69–78. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Pescovitz M. D., Hsu P. L. Monoclonal antibodies against SU-DHL-1 cells stain the neoplastic cells in true histiocytic lymphoma, malignant histiocytosis, and Hodgkin's disease. Blood. 1986 Jul;68(1):213–219. [PubMed] [Google Scholar]
- Hsu S. M. Phenotypic expression of cells of stationary elements in human lymphoid tissues. A histochemical and immunohistochemical study. Hematol Pathol. 1987;1(1):45–56. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Yang K., Jaffe E. S. Phenotypic expression of Hodgkin's and Reed-Sternberg cells in Hodgkin's disease. Am J Pathol. 1985 Feb;118(2):209–217. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. Expression of interleukin-1 in Reed-Sternberg cells and neoplastic cells from true histiocytic malignancies. Am J Pathol. 1986 Nov;125(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X., Hsu P. L., Lok M. S. Extracellular matrix does not induce the proliferation, but promotes the differentiation, of Hodgkin's cell line HDLM-1. Am J Pathol. 1987 Apr;127(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Zhao X. The H-RS-like cells in infectious mononucleosis are transformed interdigitating reticulum cells. Am J Pathol. 1987 May;127(2):403–408. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Muramoto L., Said J. Expression of T-cell antigens on Reed-Sternberg cells in a subset of patients with nodular sclerosing and mixed cellularity Hodgkin's disease. Am J Pathol. 1988 Feb;130(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Kadin M. E., Sako D., Berliner N., Franklin W., Woda B., Borowitz M., Ireland K., Schweid A., Herzog P., Lange B. Childhood Ki-1 lymphoma presenting with skin lesions and peripheral lymphadenopathy. Blood. 1986 Nov;68(5):1042–1049. [PubMed] [Google Scholar]
- Kadin M., Nasu K., Sako D., Said J., Vonderheid E. Lymphomatoid papulosis. A cutaneous proliferation of activated helper T cells expressing Hodgkin's disease-associated antigens. Am J Pathol. 1985 May;119(2):315–325. [PMC free article] [PubMed] [Google Scholar]
- Kantarjian H. M., Talpaz M., Gutterman J. U. Chronic myelogenous leukemia--past, present, and future. Hematol Pathol. 1988;2(2):91–120. [PubMed] [Google Scholar]
- Linch D. C., Jones H. M., Berliner N., MacLennan K., O'Flynn K., Huehns E. R., Kay L. A., Goff K. Hodgkin-cell leukaemia of B-cell origin. Lancet. 1985 Jan 12;1(8420):78–80. doi: 10.1016/s0140-6736(85)91968-3. [DOI] [PubMed] [Google Scholar]
- Poppema S., De Jong B., Atmosoerodjo J., Idenburg V., Visser L., De Ley L. Morphologic, immunologic, enzymehistochemical and chromosomal analysis of a cell line derived from Hodgkin's disease. Evidence for a B-cell origin of Sternberg-Reed cells. Cancer. 1985 Feb 15;55(4):683–690. doi: 10.1002/1097-0142(19850215)55:4<683::aid-cncr2820550402>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Pui C. H., Dahl G. V., Melvin S., Williams D. L., Peiper S., Mirro J., Murphy S. B., Stass S. Acute leukaemia with mixed lymphoid and myeloid phenotype. Br J Haematol. 1984 Jan;56(1):121–130. doi: 10.1111/j.1365-2141.1984.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Radzun H. J., Parwaresch M. R., Feller A. C., Hansmann M. L. Monocyte/macrophage-specific monoclonal antibody Ki-M1 recognizes interdigitating reticulum cells. Am J Pathol. 1984 Dec;117(3):441–450. [PMC free article] [PubMed] [Google Scholar]
- Robinson M. A., Kindt T. J. B lymphoblastoid cell lines with rearranged T cell receptor beta-chain genes retain conventional B cell surface antigen and Ig expression. J Immunol. 1988 Feb 15;140(4):1327–1334. [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stein H., Mason D. Y., Gerdes J., O'Connor N., Wainscoat J., Pallesen G., Gatter K., Falini B., Delsol G., Lemke H. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985 Oct;66(4):848–858. [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Takuma T., Takeda K., Konno K. Synergism of tumor necrosis factor and interferon-gamma in induction of differentiation of human myeloblastic leukemic ML-1 cells. Biochem Biophys Res Commun. 1987 May 29;145(1):514–521. doi: 10.1016/0006-291x(87)91351-9. [DOI] [PubMed] [Google Scholar]
- Tobler A., Johnston D., Koeffler H. P. Recombinant human tumor necrosis factor alpha regulates c-myc expression in HL-60 cells at the level of transcription. Blood. 1987 Jul;70(1):200–205. [PubMed] [Google Scholar]
- Trinchieri G., Kobayashi M., Rosen M., Loudon R., Murphy M., Perussia B. Tumor necrosis factor and lymphotoxin induce differentiation of human myeloid cell lines in synergy with immune interferon. J Exp Med. 1986 Oct 1;164(4):1206–1225. doi: 10.1084/jem.164.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Winter J. N., Variakojis D., Epstein A. L. Phenotypic analysis of established diffuse histiocytic lymphoma cell lines utilizing monoclonal antibodies and cytochemical techniques. Blood. 1984 Jan;63(1):140–146. [PubMed] [Google Scholar]
- Woolford J., Rothwell V., Rohrschneider L. Characterization of the human c-fms gene product and its expression in cells of the monocyte-macrophage lineage. Mol Cell Biol. 1985 Dec;5(12):3458–3466. doi: 10.1128/mcb.5.12.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]