Abstract
Opioid peptides have a variety of actions on inter alia pituitary hormone secretion and the immune system. Release of endogenous opioids has been found to stimulate growth of experimental breast cancers and opiate receptor blockers have reduced the growth of chemically induced rat breast tumors. Opioid peptides may therefore play a role in human breast cancer. Invasive ductal carcinomas from 61 premenopausal women were immunocytochemically analyzed for the presence of opioid peptide immunoreactivity. Positive staining was unambiguously identified in 34 of the tumors (56%). In addition, a medullary carcinoma was positive. In a smaller series of tumors, opioid peptide immunoreactive cells were detected in both primary tumors and metastases. Positive tumor cells were usually few and scattered. Therefore, underestimates of their true frequency of occurrence are likely to have occurred, making accurate correlations with clinical behavior and estrogen receptor status difficult. No correlations with estrogen receptors were established for the unambiguously opioid peptide-positive tumors. Many of the positive tumors also stained with antibodies to gamma-endorphin and alpha-melanocyte-stimulating hormone, suggesting the presence of proopiomelanocortin-derived peptides in them. However, peptides derived from other opioid precursors also may be present in breast cancer.
Full text
PDF

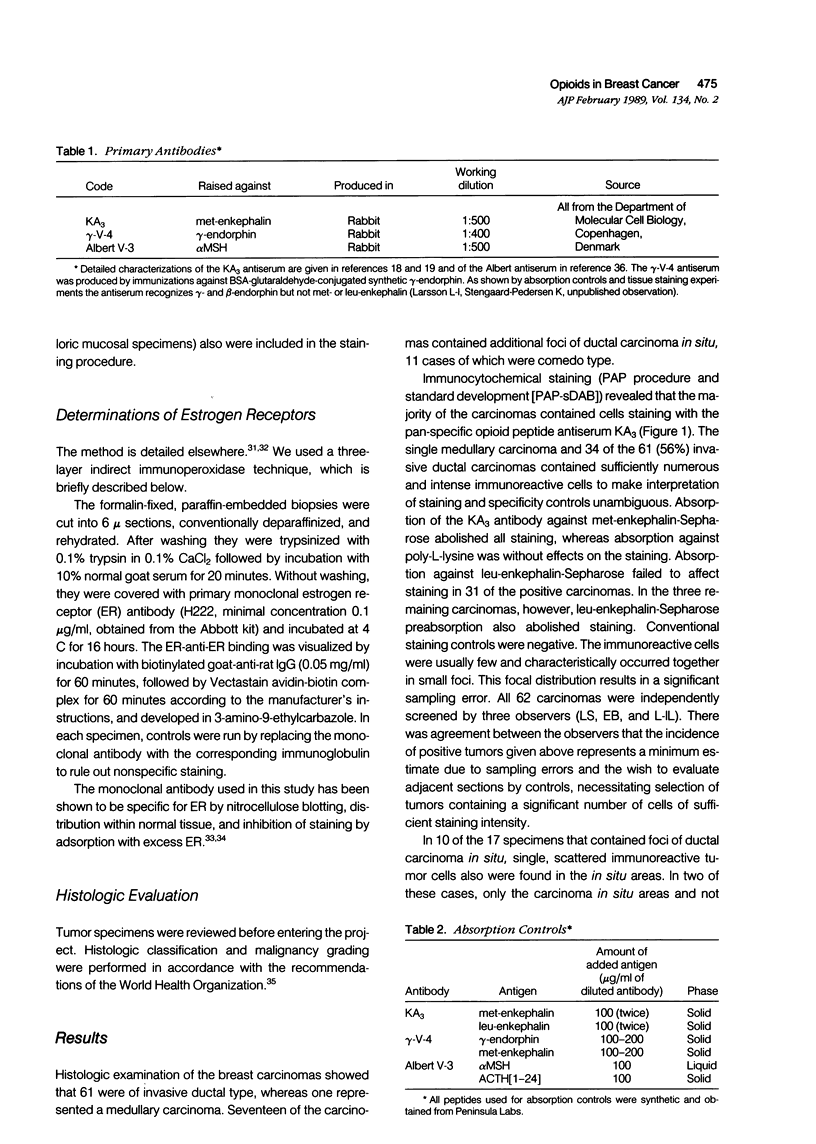
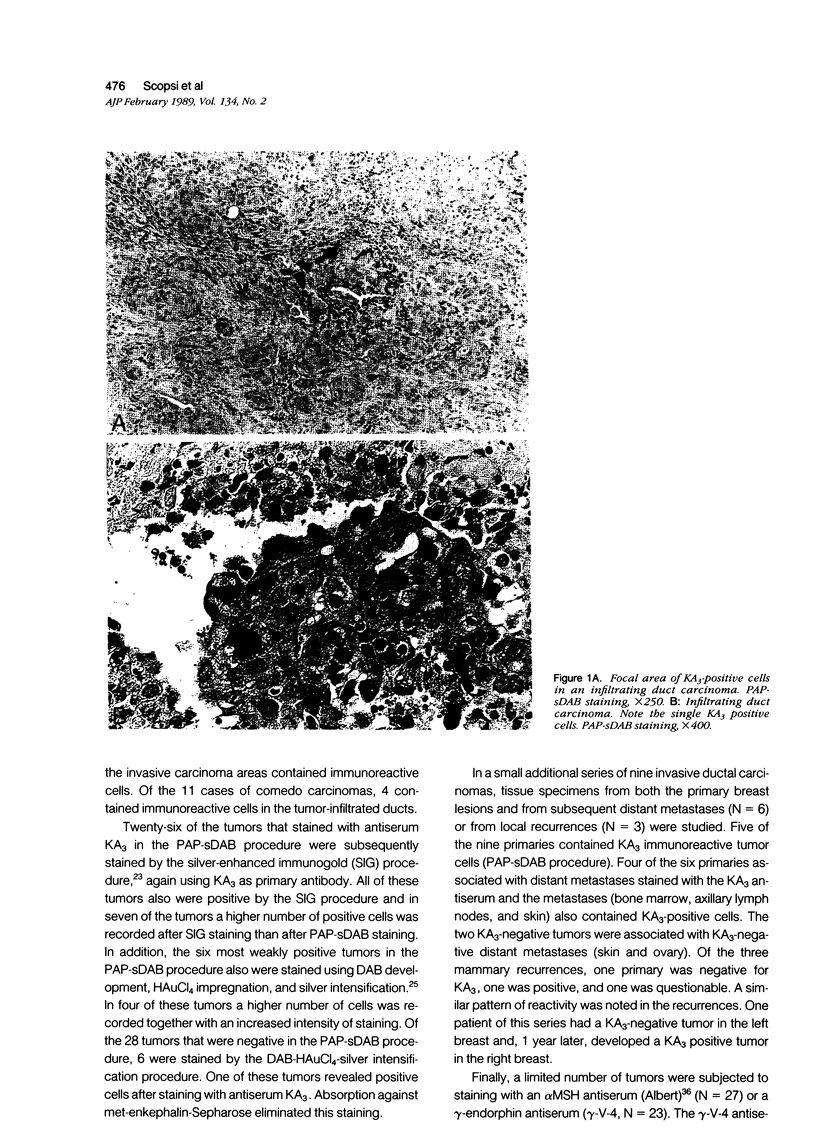



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J., Orntoft T. F., Andersen J. A., Poulsen H. S. Gynecomastia. Immunohistochemical demonstration of estrogen receptors. Acta Pathol Microbiol Immunol Scand A. 1987 Sep;95(5):263–267. doi: 10.1111/j.1699-0463.1987.tb00040_95a.x. [DOI] [PubMed] [Google Scholar]
- Andersen K. W., Mouridsen H. T., Castberg T., Fischerman K., Andersen J., Hou-Jensen K., Brincker H., Johansen H., Henriksen E., Rørth M. Organisation of the Danish adjuvant trials in breast cancer. Dan Med Bull. 1981 Aug;28(3):102–106. [PubMed] [Google Scholar]
- Aylsworth C. F., Hodson C. A., Meites J. Opiate antagonists can inhibit mammary tumor growth in rats. Proc Soc Exp Biol Med. 1979 May;161(1):18–20. doi: 10.3181/00379727-161-40479. [DOI] [PubMed] [Google Scholar]
- Blalock J. E. Proopiomelanocortin-derived peptides in the immune system. Clin Endocrinol (Oxf) 1985 Jun;22(6):823–827. doi: 10.1111/j.1365-2265.1985.tb00173.x. [DOI] [PubMed] [Google Scholar]
- Brantl V., Teschemacher H., Henschen A., Lottspeich F. Novel opioid peptides derived from casein (beta-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers Z Physiol Chem. 1979 Sep;360(9):1211–1216. doi: 10.1515/bchm2.1979.360.2.1211. [DOI] [PubMed] [Google Scholar]
- Bronzert D. A., Pantazis P., Antoniades H. N., Kasid A., Davidson N., Dickson R. B., Lippman M. E. Synthesis and secretion of platelet-derived growth factor by human breast cancer cell lines. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5763–5767. doi: 10.1073/pnas.84.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Azzopardi J. G., Krausz T., van Noorden S., Polak J. M. A morphological and immunocytochemical study of a distinctive variant of ductal carcinoma in-situ of the breast. Histopathology. 1985 Jan;9(1):21–37. doi: 10.1111/j.1365-2559.1985.tb02968.x. [DOI] [PubMed] [Google Scholar]
- Danscher G. Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electronmicroscopy. Histochemistry. 1981;71(1):1–16. doi: 10.1007/BF00592566. [DOI] [PubMed] [Google Scholar]
- Dixon W. R., Viveros O. H., Unsworth C. D., Diliberto E. J., Jr, Way E. L., Lewis J. W., Chandra A., Holaday J. W., Long J. B., Tortella F. C. Multiple opiate receptors: functional implications. Fed Proc. 1985 Oct;44(13):2851–2862. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Henschen A., Lottspeich F., Brantl V., Teschemacher H. Novel opioid peptides derived from casein (beta-casomorphins). II. Structure of active components from bovine casein peptone. Hoppe Seylers Z Physiol Chem. 1979 Sep;360(9):1217–1224. [PubMed] [Google Scholar]
- Holgate C. S., Jackson P., Cowen P. N., Bird C. C. Immunogold-silver staining: new method of immunostaining with enhanced sensitivity. J Histochem Cytochem. 1983 Jul;31(7):938–944. doi: 10.1177/31.7.6189883. [DOI] [PubMed] [Google Scholar]
- Howlett T. A., Rees L. H. Endogenous opioid peptides and hypothalamo-pituitary function. Annu Rev Physiol. 1986;48:527–536. doi: 10.1146/annurev.ph.48.030186.002523. [DOI] [PubMed] [Google Scholar]
- Huff K. K., Kaufman D., Gabbay K. H., Spencer E. M., Lippman M. E., Dickson R. B. Secretion of an insulin-like growth factor-I-related protein by human breast cancer cells. Cancer Res. 1986 Sep;46(9):4613–4619. [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Larsson L. I. Adrenocorticotropin-like and alpha-melanotropin-like peptides in a subpopulation of human gastrin cell granules: bioassay, immunoassay, and immunocytochemical evidence. Proc Natl Acad Sci U S A. 1981 May;78(5):2990–2994. doi: 10.1073/pnas.78.5.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. I. Peptide immunocytochemistry. Prog Histochem Cytochem. 1981;13(4):1–85. [PubMed] [Google Scholar]
- Larsson L. I., Stengaard-Pedersen K. Enkephalin/endorphin-related peptides in antropyloric gastrin cells. J Histochem Cytochem. 1981 Sep;29(9):1088–1098. doi: 10.1177/29.9.6116733. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Stengaard-Pendersen K. Immunocytochemical and ultrastructural differentiation between Met-enkephalin-, Leu-enkephalin-, and Met/Leu-enkephalin-immunoreactive neurons of feline gut. J Neurosci. 1982 Jul;2(7):861–878. doi: 10.1523/JNEUROSCI.02-07-00861.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. W., Shavit Y., Terman G. W., Nelson L. R., Gale R. P., Liebeskind J. C. Apparent involvement of opioid peptides in stress-induced enhancement of tumor growth. Peptides. 1983 Sep-Oct;4(5):635–638. doi: 10.1016/0196-9781(83)90010-4. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., Clements J. A., Markwick A. J., Cheng C., McNally M., Smith A. I., Funder J. W. Pro-opiomelanocortin messenger ribonucleic acid and posttranslational processing of beta endorphin in spleen macrophages. J Clin Invest. 1986 Jun;77(6):1776–1779. doi: 10.1172/JCI112501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait S. J., Lim A. T., Toh B. H., Funder J. W. Immunoreactive beta-endorphin in a subpopulation of mouse spleen macrophages. J Clin Invest. 1984 Jan;73(1):277–280. doi: 10.1172/JCI111203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lottspeich F., Henschen A., Brantl V., Teschemacher H. Novel opioid peptides derived from casein (beta-casomorphins). III. Synthetic peptides corresponding to components from bovine casein peptone. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1835–1839. doi: 10.1515/bchm2.1980.361.2.1835. [DOI] [PubMed] [Google Scholar]
- Mendelson C. R., Smith M. E., Cleland W. H., Simpson E. R. Regulation of aromatase activity of cultured adipose stromal cells by catecholamines and adrenocorticotropin. Mol Cell Endocrinol. 1984 Aug;37(1):61–72. doi: 10.1016/0303-7207(84)90128-x. [DOI] [PubMed] [Google Scholar]
- Meyer W. J., 3rd, Smith E. M., Richards G. E., Cavallo A., Morrill A. C., Blalock J. E. In vivo immunoreactive adrenocorticotropin (ACTH) production by human mononuclear leukocytes from normal and ACTH-deficient individuals. J Clin Endocrinol Metab. 1987 Jan;64(1):98–105. doi: 10.1210/jcem-64-1-98. [DOI] [PubMed] [Google Scholar]
- Mori K., Kurobe M., Furukawa S., Kubo K., Hayashi K. Human breast cancer cells synthesize and secrete an EGF-like immunoreactive factor in culture. Biochem Biophys Res Commun. 1986 Apr 14;136(1):300–305. doi: 10.1016/0006-291x(86)90909-5. [DOI] [PubMed] [Google Scholar]
- Morley J. E. Neuroendocrine effects of endogenous opioid peptides in human subjects: a review. Psychoneuroendocrinology. 1983;8(4):361–379. doi: 10.1016/0306-4530(83)90016-1. [DOI] [PubMed] [Google Scholar]
- Nesland J. M., Holm R., Johannessen J. V., Gould V. E. Neurone specific enolase immunostaining in the diagnosis of breast carcinomas with neuroendocrine differentiation. Its usefulness and limitations. J Pathol. 1986 Jan;148(1):35–43. doi: 10.1002/path.1711480107. [DOI] [PubMed] [Google Scholar]
- Nesland J. M., Lunde S., Holm R., Johannessen J. V. Electron microscopy and immunostaining of the normal breast and its benign lesions. A search for neuroendocrine cells. Histol Histopathol. 1987 Jan;2(1):73–77. [PubMed] [Google Scholar]
- Nesland J. M., Memoli V. A., Holm R., Gould V. E., Johannessen J. V. Breast carcinomas with neuroendocrine differentiation. Ultrastruct Pathol. 1985;8(2-3):225–240. doi: 10.3109/01913128509142155. [DOI] [PubMed] [Google Scholar]
- Olson G. A., Olson R. D., Kastin A. J. Endogenous opiates: 1985. Peptides. 1986 Sep-Oct;7(5):907–933. doi: 10.1016/0196-9781(86)90113-0. [DOI] [PubMed] [Google Scholar]
- Press M. F., Greene G. L. An immunocytochemical method for demonstrating estrogen receptor in human uterus using monoclonal antibodies to human estrophilin. Lab Invest. 1984 Apr;50(4):480–486. [PubMed] [Google Scholar]
- Scopsi L., Larsson L. I. Increased sensitivity in peroxidase immunocytochemistry. A comparative study of a number of peroxidase visualization methods employing a model system. Histochemistry. 1986;84(3):221–230. doi: 10.1007/BF00495786. [DOI] [PubMed] [Google Scholar]
- Scopsi L., Wang B. L., Larsson L. I. Nonspecific immunocytochemical reactions with certain neurohormonal peptides and basic peptide sequences. J Histochem Cytochem. 1986 Nov;34(11):1469–1475. doi: 10.1177/34.11.2877024. [DOI] [PubMed] [Google Scholar]
- Shavit Y., Depaulis A., Martin F. C., Terman G. W., Pechnick R. N., Zane C. J., Gale R. P., Liebeskind J. C. Involvement of brain opiate receptors in the immune-suppressive effect of morphine. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7114–7117. doi: 10.1073/pnas.83.18.7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Smith E. M., Morrill A. C., Meyer W. J., 3rd, Blalock J. E. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. 1986 Jun 26-Jul 2Nature. 321(6073):881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Vértes M., Pámer Z., Garai J. On the mechanism of opioid-oestradiol interactions. J Steroid Biochem. 1986 Jan;24(1):235–238. doi: 10.1016/0022-4731(86)90056-7. [DOI] [PubMed] [Google Scholar]
- Wang B. L., Scopsi L., Hartvig Nielsen M., Larsson L. I. Simplified purification and testing of colloidal gold probes. Histochemistry. 1985;83(2):109–115. doi: 10.1007/BF00495139. [DOI] [PubMed] [Google Scholar]
- Westly H. J., Kleiss A. J., Kelley K. W., Wong P. K., Yuen P. H. Newcastle disease virus-infected splenocytes express the proopiomelanocortin gene. J Exp Med. 1986 Jun 1;163(6):1589–1594. doi: 10.1084/jem.163.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J., Goodman S. R., Rhodes R. E. Opioid receptors and endogenous opioids in diverse human and animal cancers. J Natl Cancer Inst. 1987 Nov;79(5):1059–1065. [PubMed] [Google Scholar]
- Zagon I. S., McLaughlin P. J. Stereospecific modulation of tumorigenicity by opioid antagonists. Eur J Pharmacol. 1985 Jul 11;113(1):115–120. doi: 10.1016/0014-2999(85)90350-4. [DOI] [PubMed] [Google Scholar]