Abstract
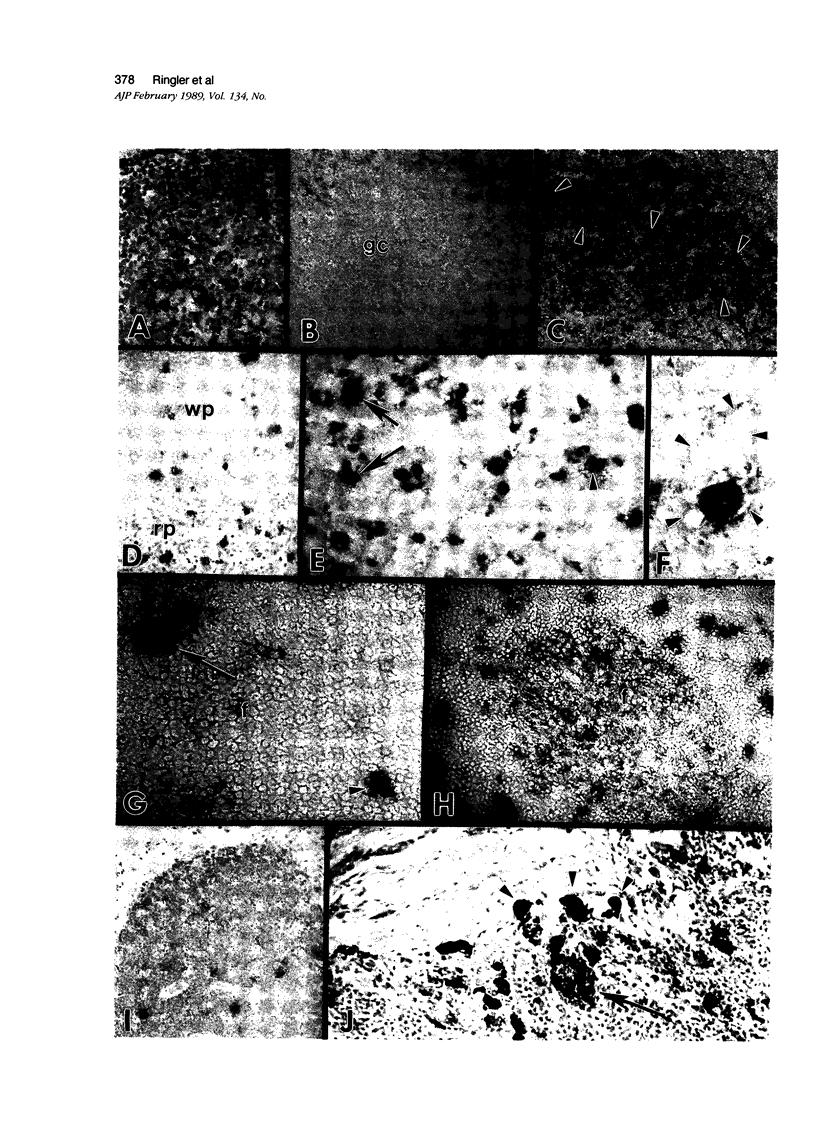
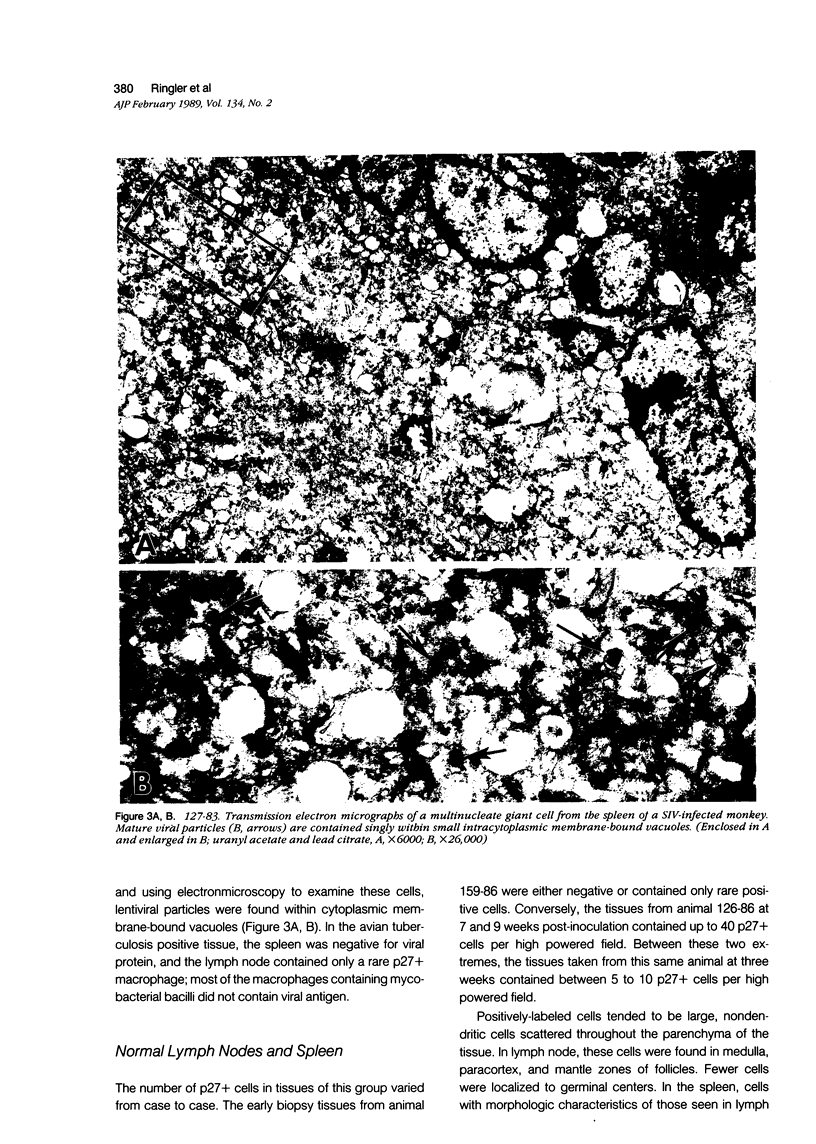
Simian immunodeficiency virus (SIV) is a lentivirus with genetic relatedness to the human immunodeficiency viruses (HIV-1 and HIV-2). It induces a fatal syndrome in rhesus monkeys that closely parallels the clinical course of AIDS in humans. The authors used double-labeling immunohistochemical procedures on rhesus lymph node and spleen taken during different time periods after SIV infection to localize the p27 gag protein to specific cellular immunophenotypes. In animals with follicular hyperplasia, viral protein was found associated predominantly with follicular dendritic cells. Many of these cells showed ultrastructural alterations consisting of swollen dendritic processes contaning electron-dense material. Lentiviral particles were found associated with this cell type only rarely. In lymphoid tissues with other histopathologic changes, macrophages and multinucleate giant cells were the predominant cell types containing detectable quantities of viral protein; smaller numbers of p27+ lymhocytes were present. Ultrastructurally, viral particles were found within the extracellular spce adjacent to tissue macrophages and within membrane-bound vacuoles of giant cells and tissue macrophage. These results show that certain histologic patterns seen during the course of infection correlate with the localization of viral antigen to specific cellular immunophenotypes and that during the disease course, viral protein is preferentially localized in sections of lymphonode and spleen to cells of the macrophage and dendritic cell lineage.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Horne R. Follicular dendritic cells and virus-like particles in AIDS-related lymphadenopathy. Lancet. 1984 Aug 18;2(8399):370–372. doi: 10.1016/s0140-6736(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Baroni C. D., Pezzella F., Mirolo M., Ruco L. P., Rossi G. B. Immunohistochemical demonstration of p24 HTLV III major core protein in different cell types within lymph nodes from patients with lymphadenopathy syndrome (LAS). Histopathology. 1986 Jan;10(1):5–13. doi: 10.1111/j.1365-2559.1986.tb02456.x. [DOI] [PubMed] [Google Scholar]
- Biberfeld P., Chayt K. J., Marselle L. M., Biberfeld G., Gallo R. C., Harper M. E. HTLV-III expression in infected lymph nodes and relevance to pathogenesis of lymphadenopathy. Am J Pathol. 1986 Dec;125(3):436–442. [PMC free article] [PubMed] [Google Scholar]
- Biberfeld P., Porwit-Ksiazek A., Böttiger B., Morfeldt-Månsson L., Biberfeld G. Immunohistopathology of lymph nodes in HTLV-III infected homosexuals with persistent adenopathy or AIDS. Cancer Res. 1985 Sep;45(9 Suppl):4665s–4670s. [PubMed] [Google Scholar]
- Bulman A. S., Heyderman E. Alkaline phosphatase for immunocytochemical labelling: problems with endogenous enzyme activity. J Clin Pathol. 1981 Dec;34(12):1349–1351. doi: 10.1136/jcp.34.12.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chalifoux L. V., King N. W., Daniel M. D., Kannagi M., Desrosiers R. C., Sehgal P. K., Waldron L. M., Hunt R. D., Letvin N. L. Lymphoproliferative syndrome in an immunodeficient rhesus monkey naturally infected with an HTLV-III-like virus (STLV-III). Lab Invest. 1986 Jul;55(1):43–50. [PubMed] [Google Scholar]
- Chalifoux L. V., King N. W., Letvin N. L. Morphologic changes in lymph nodes of macaques with an immunodeficiency syndrome. Lab Invest. 1984 Jul;51(1):22–26. [PubMed] [Google Scholar]
- Chalifoux L. V., Ringler D. J., King N. W., Sehgal P. K., Desrosiers R. C., Daniel M. D., Letvin N. L. Lymphadenopathy in macaques experimentally infected with the simian immunodeficiency virus (SIV). Am J Pathol. 1987 Jul;128(1):104–110. [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Schmidt D. K., Silva D. P., Solomon K. R., Hodi F. S., Jr, Ringler D. J., Hunt R. D., King N. W. Prevalence of antibodies to 3 retroviruses in a captive colony of macaque monkeys. Int J Cancer. 1988 Apr 15;41(4):601–608. doi: 10.1002/ijc.2910410421. [DOI] [PubMed] [Google Scholar]
- Diebold J., Marche C., Audouin J., Aubert J. P., Le Tourneau A., Bouton C., Reynes M., Wizniak J., Capron F., Tricottet V. Lymph node modification in patients with the acquired immunodeficiency syndrome (AIDS) or with AIDS related complex (ARC). A histological, immuno-histopathological and ultrastructural study of 45 cases. Pathol Res Pract. 1985 Dec;180(6):590–611. doi: 10.1016/S0344-0338(85)80037-6. [DOI] [PubMed] [Google Scholar]
- Friedman-Kien A. E., Laubenstein L. J., Rubinstein P., Buimovici-Klein E., Marmor M., Stahl R., Spigland I., Kim K. S., Zolla-Pazner S. Disseminated Kaposi's sarcoma in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):693–700. doi: 10.7326/0003-4819-96-6-693. [DOI] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Molineaux S., Clements J. E., Ghotbi Z. Slow, persistent replication of lentiviruses: role of tissue macrophages and macrophage precursors in bone marrow. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7086–7090. doi: 10.1073/pnas.82.20.7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock W. W., Atkins R. C. Immunohistologic analysis of the cell surface antigens of human dendritic cells using monoclonal antibodies. Transplant Proc. 1984 Aug;16(4):963–967. [PubMed] [Google Scholar]
- Hancock W. W., Becker G. J., Atkins R. C. A comparison of fixatives and immunohistochemical technics for use with monoclonal antibodies to cell surface antigens. Am J Clin Pathol. 1982 Dec;78(6):825–831. doi: 10.1093/ajcp/78.6.825. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Lord R. H., Colby A. J., Diamantstein T., Rickles F. R., Dijkstra C., Hogg N., Tilney N. L. Identification of IL 2R+ T cells and macrophages within rejecting rat cardiac allografts, and comparison of the effects of treatment with anti-IL 2R monoclonal antibody or cyclosporin. J Immunol. 1987 Jan 1;138(1):164–170. [PubMed] [Google Scholar]
- Hanna M. G., Jr, Szakal A. K. Localization of 125I-labeled antigen in germinal centers of mouse spleen: histologic and ultrastructural autoradiographic studies of the secondary immune reaction. J Immunol. 1968 Nov;101(5):949–962. [PubMed] [Google Scholar]
- Kanki P. J., McLane M. F., King N. W., Jr, Letvin N. L., Hunt R. D., Sehgal P., Daniel M. D., Desrosiers R. C., Essex M. Serologic identification and characterization of a macaque T-lymphotropic retrovirus closely related to HTLV-III. Science. 1985 Jun 7;228(4704):1199–1201. doi: 10.1126/science.3873705. [DOI] [PubMed] [Google Scholar]
- Kannagi M., Kiyotaki M., Desrosiers R. C., Reimann K. A., King N. W., Waldron L. M., Letvin N. L. Humoral immune responses to T cell tropic retrovirus simian T lymphotropic virus type III in monkeys with experimentally induced acquired immune deficiency-like syndrome. J Clin Invest. 1986 Nov;78(5):1229–1236. doi: 10.1172/JCI112706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi M., Yetz J. M., Letvin N. L. In vitro growth characteristics of simian T-lymphotropic virus type III. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7053–7057. doi: 10.1073/pnas.82.20.7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Barré-Sinoussi F., Nugeyre M. T., Danquet C., Vilmer E., Griscelli C., Brun-Veziret F., Rouzioux C., Gluckman J. C., Chermann J. C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984 Jul 6;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Koenig S., Gendelman H. E., Orenstein J. M., Dal Canto M. C., Pezeshkpour G. H., Yungbluth M., Janotta F., Aksamit A., Martin M. A., Fauci A. S. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986 Sep 5;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Daniel M. D., Sehgal P. K., Desrosiers R. C., Hunt R. D., Waldron L. M., MacKey J. J., Schmidt D. K., Chalifoux L. V., King N. W. Induction of AIDS-like disease in macaque monkeys with T-cell tropic retrovirus STLV-III. Science. 1985 Oct 4;230(4721):71–73. doi: 10.1126/science.2412295. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- Mildvan D., Mathur U., Enlow R. W., Romain P. L., Winchester R. J., Colp C., Singman H., Adelsberg B. R., Spigland I. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- Minassian A. A., Kalyanaraman V. S., Gallo R. C., Popovic M. Monoclonal antibodies against human immunodeficiency virus (HIV) type 2 core proteins: cross-reactivity with HIV type 1 and simian immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6939–6943. doi: 10.1073/pnas.85.18.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L., Distenfeld A., Amorosi E., Karpatkin S. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982 Jun;96(6 Pt 1):714–717. doi: 10.7326/0003-4819-96-6-714. [DOI] [PubMed] [Google Scholar]
- Narayan O., Kennedy-Stoskopf S., Sheffer D., Griffin D. E., Clements J. E. Activation of caprine arthritis-encephalitis virus expression during maturation of monocytes to macrophages. Infect Immun. 1983 Jul;41(1):67–73. doi: 10.1128/iai.41.1.67-73.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan O., Wolinsky J. S., Clements J. E., Strandberg J. D., Griffin D. E., Cork L. C. Slow virus replication: the role of macrophages in the persistence and expression of visna viruses of sheep and goats. J Gen Virol. 1982 Apr;59(Pt 2):345–356. doi: 10.1099/0022-1317-59-2-345. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Abbot A., Mitchell J., Lummus Z. Antigens in immunity. XV. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968 Feb 1;127(2):277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Knight S. C. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987 Apr;68(Pt 4):1177–1181. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- Ringler D. J., Hancock W. W., King N. W., Letvin N. L., Daniel M. D., Desrosiers R. C., Murphy G. F. Immunophenotypic characterization of the cutaneous exanthem of SIV-infected rhesus monkeys. Apposition of degenerative Langerhans cells and cytotoxic lymphocytes during the development of acquired immunodeficiency syndrome. Am J Pathol. 1987 Feb;126(2):199–207. [PMC free article] [PubMed] [Google Scholar]
- Ringler D. J., Hunt R. D., Desrosiers R. C., Daniel M. D., Chalifoux L. V., King N. W. Simian immunodeficiency virus-induced meningoencephalitis: natural history and retrospective study. Ann Neurol. 1988;23 (Suppl):S101–S107. doi: 10.1002/ana.410230726. [DOI] [PubMed] [Google Scholar]
- Ringler D. J., Walsh D. G., MacKey J. J., Hunt R. D., King N. W. Immunophenotypic characterization of mononuclear phagocytes and dendritic cells in lymphoid organs of the rhesus monkey. Clin Immunol Immunopathol. 1988 Dec;49(3):349–364. doi: 10.1016/0090-1229(88)90125-0. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Rose R. M., Groopman J. E., Markham P. D., Gallo R. C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986 Jul;68(1):281–284. [PubMed] [Google Scholar]
- Tenner-Racz K., Racz P., Bofill M., Schulz-Meyer A., Dietrich M., Kern P., Weber J., Pinching A. J., Veronese-Dimarzo F., Popovic M. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986 Apr;123(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Tenner-Rácz K., Rácz P., Dietrich M., Kern P. Altered follicular dendritic cells and virus-like particles in AIDS and AIDS-related lymphadenopathy. Lancet. 1985 Jan 12;1(8420):105–106. doi: 10.1016/s0140-6736(85)91994-4. [DOI] [PubMed] [Google Scholar]
- Ward J. M., O'Leary T. J., Baskin G. B., Benveniste R., Harris C. A., Nara P. L., Rhodes R. H. Immunohistochemical localization of human and simian immunodeficiency viral antigens in fixed tissue sections. Am J Pathol. 1987 May;127(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Burns B. F., Dorfman R. F., Warnke R. A. In situ quantitation of lymph node helper, suppressor, and cytotoxic T cell subsets in AIDS. Blood. 1986 Mar;67(3):596–603. [PubMed] [Google Scholar]
- Wood G. S., Turner R. R., Shiurba R. A., Eng L., Warnke R. A. Human dendritic cells and macrophages. In situ immunophenotypic definition of subsets that exhibit specific morphologic and microenvironmental characteristics. Am J Pathol. 1985 Apr;119(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]
- Wyand M. S., Ringler D. J., Naidu Y. M., Mattmuller M., Chalifoux L. V., Sehgal P. K., Daniel M. D., Desrosiers R. C., King N. W. Cellular localization of simian immunodeficiency virus in lymphoid tissues. II. In situ hybridization. Am J Pathol. 1989 Feb;134(2):385–393. [PMC free article] [PubMed] [Google Scholar]