Abstract
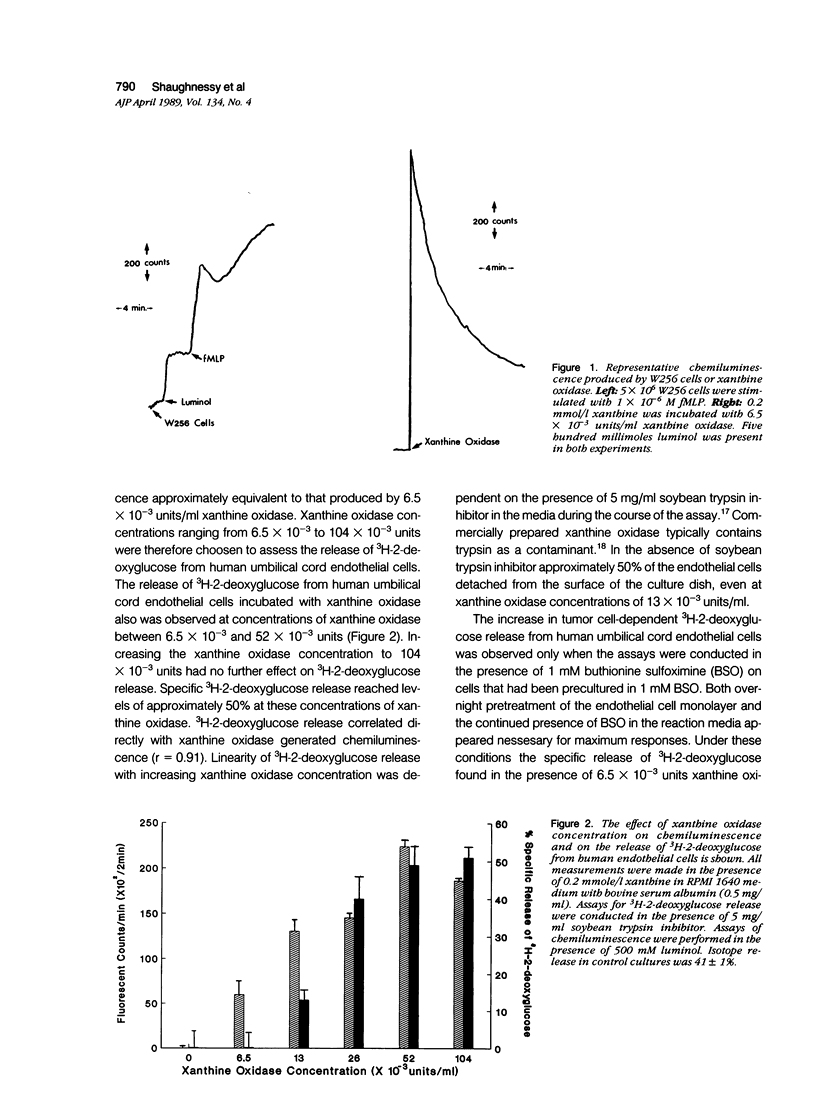
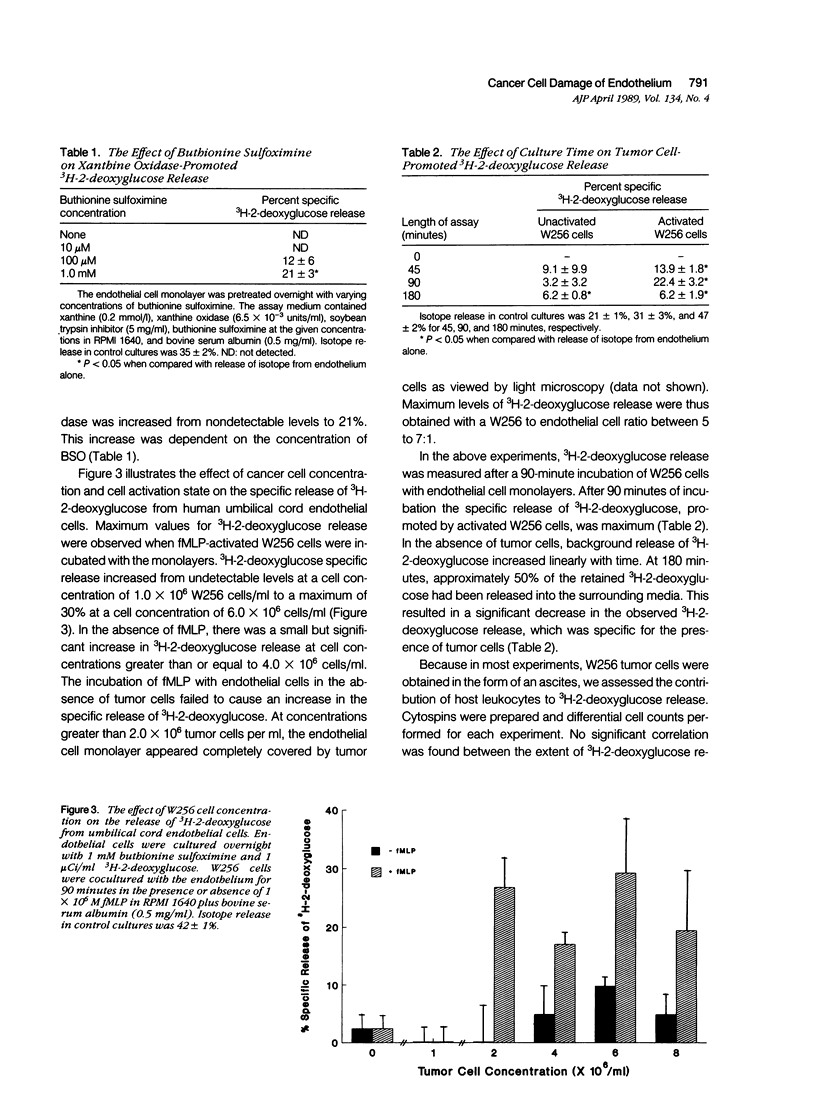
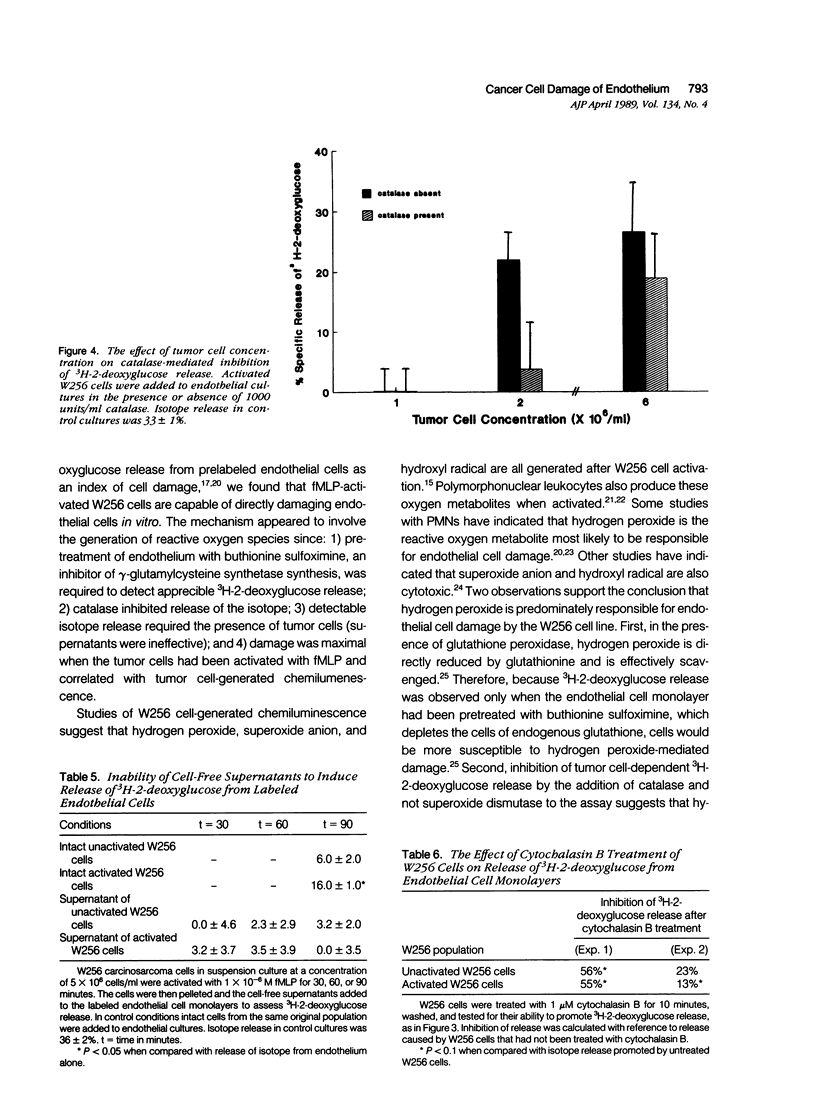
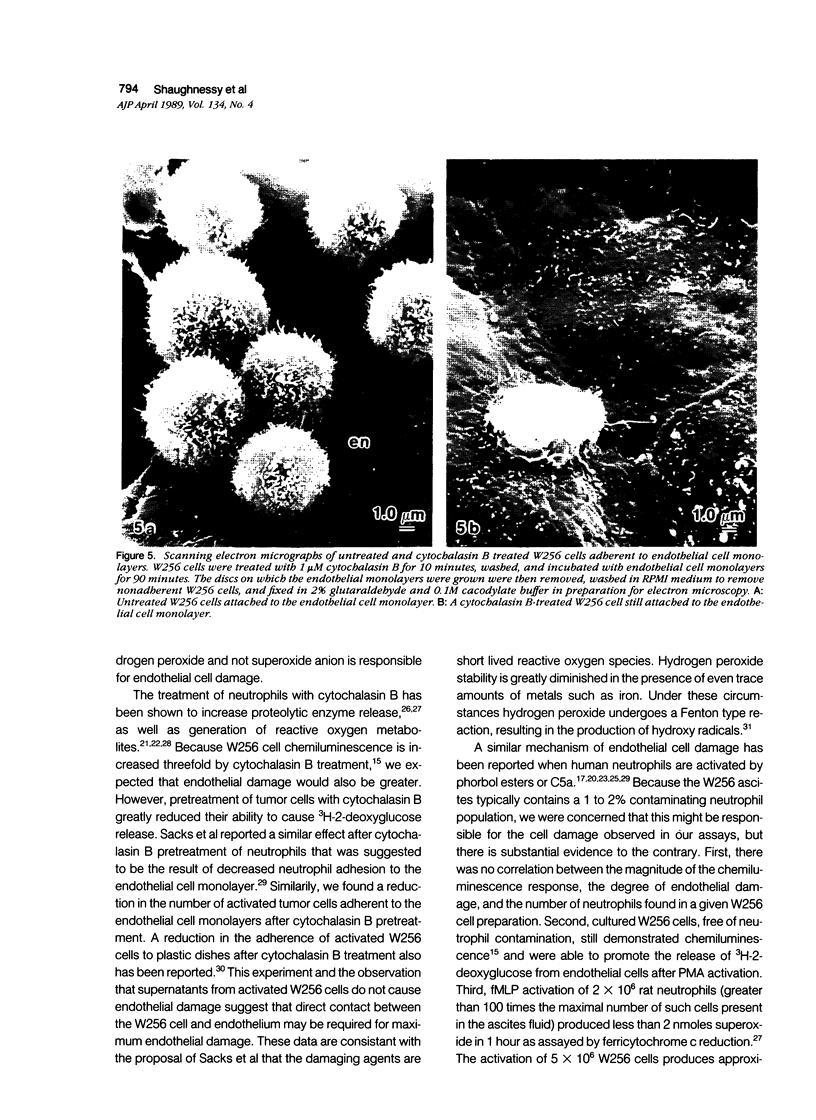
The passage of circulating tumor cells across vessel walls is an important step in cancer metastasis and is promoted by endothelial injury. Because Walker carcinosarcoma 256 (W256) cells generate oxygen-derived free radicals after cellular activation, the authors tested the hypothesis that these cancer cells can damage endothelial monolayers by producing such reactive oxygen species. To confirm that oxygen-derived radicals can damage endothelial cells, 3H-2-deoxyglucose-labeled human endothelial cell monolayers were exposed to xanthine oxidase in the presence of 0.2 mmol/l xanthine. 3H-2-deoxyglucose release was observed after the addition of xanthine oxidase in concentrations ranging from 6.5 x 10(-3) to 52 x 10(-3) units/ml. The extent of damage correlated with xanthine oxidase-dependent chemiluminescence (r = 0.91). Chemiluminescence assays in the presence of 5 x 10(-5) M luminol confirmed activation of the W256 cells by 1 x 10(-6) M chemotactic peptide fMLP. When fMLP-activated activated W256 cells were incubated with endothelial monolayers, concentrations of 2 x 10(6) to 6 x 10(6) W256 cells/ml were found to cause a 27% increase in the specific release of 2-deoxyglucose after a 90-minute incubation. A small but significant increase in 3H-2-deoxyglucose release also was observed in the absence of fMLP. Detection of 3H-2-deoxyglucose release in the presence of activated or unactivated tumor cells was dependent on preincubating the endothelial cell monolayer with 1 mM buthionine sulfoximine, an inhibitor of glutathione synthesis. Under these conditions, the specific release of 3H-2-deoxyglucose was increased from nondetectable levels to 21%, in the presence of 6.5 x 10(-3) units of the oxidase. Cultured W256 cells promoted isotope release from endothelial cell monolayers when activated with phorbol myristate acetate. Catalase (1000 units/ml) inhibited the tumor cell-induced release of 3H-2-deoxyglucose by 84% whereas superoxide dismutase, even at concentrations of 1 mg/ml, had no effect. A requirement for cell contact was shown because addition of cell-free supernatants from fMLP activated tumor cells did not cause 3H-2-deoxyglucose release and because pretreatment of W256 cells with 1 microM cytochalasin B inhibited their ability to promote isotope release even while increasing tumor cell-generated chemiluminescence threefold. Electron microscopy revealed that fewer cytochalasin B-treated W256 cells were attached to the endothelial cell monolayer than in untreated controls. It is concluded that the W256 tumor cells can damage endothelial cells directly via a mechanism involving production of reactive oxygen species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Orr F. W., Young L. Effects of injury and repair of the pulmonary endothelium on lung metastasis after bleomycin. J Pathol. 1986 Dec;150(4):279–287. doi: 10.1002/path.1711500407. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Young L., Orr F. W. Tumor metastasis after hyperoxic injury and repair of the pulmonary endothelium. Lab Invest. 1987 Jul;57(1):71–77. [PubMed] [Google Scholar]
- Ager A., Wenham D. J., Gordon J. L. Stimulation of endothelial cells by protease activity in commercial preparations of xanthine oxidase. Thromb Res. 1984 Jul 1;35(1):43–52. doi: 10.1016/0049-3848(84)90311-6. [DOI] [PubMed] [Google Scholar]
- Andreoli S. P., Baehner R. L., Bergstein J. M. In vitro detection of endothelial cell damage using 2-deoxy-D-3H-glucose: comparison with chromium 51, 3H-leucine, 3H-adenine, and lactate dehydrogenase. J Lab Clin Med. 1985 Sep;106(3):253–261. [PubMed] [Google Scholar]
- Andreoli S. P., Mallett C. P., Bergstein J. M. Role of glutathione in protecting endothelial cells against hydrogen peroxide oxidant injury. J Lab Clin Med. 1986 Sep;108(3):190–198. [PubMed] [Google Scholar]
- Auerbach R., Lu W. C., Pardon E., Gumkowski F., Kaminska G., Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987 Mar 15;47(6):1492–1496. [PubMed] [Google Scholar]
- BASERGA R., SAFFIOTTI U. Experimental studies on histogenesis of blood-borne metastases. AMA Arch Pathol. 1955 Jan;59(1):26–34. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Crissman J. D., Hatfield J. S., Menter D. G., Sloane B., Honn K. V. Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res. 1988 Jul 15;48(14):4065–4072. [PubMed] [Google Scholar]
- Dao T. L., Yogo H. Enhancement of pulmonary metastases by x-irradiation in rats bearing mammary cancer. Cancer. 1967 Nov;20(11):2020–2025. doi: 10.1002/1097-0142(196711)20:11<2020::aid-cncr2820201131>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Two distinct mechanisms for stimulation of oxygen-radical production by polymorphonuclear leucocytes. Biochem J. 1983 Nov 15;216(2):459–465. doi: 10.1042/bj2160459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Harker L. A., Striker G. E., Wright D. G. Neutrophil-mediated endothelial injury in vitro mechanisms of cell detachment. J Clin Invest. 1981 Dec;68(6):1394–1403. doi: 10.1172/JCI110390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M., Levine J. D., Callahan K. S., Schwartz B. R., Harker L. A. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. J Clin Invest. 1984 Mar;73(3):706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch G. E., Gardner D. E., Menzel D. B. Chemiluminescence of phagocytic cells caused by N-formylmethionyl peptides. J Exp Med. 1978 Jan 1;147(1):182–195. doi: 10.1084/jem.147.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Tanaka K. Artificial metastases and decrease of fibrinolysis in the nude mouse lung after hemithoracic irradiation. Clin Exp Metastasis. 1984 Oct-Dec;2(4):311–319. doi: 10.1007/BF00135170. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri D., Bertomeu M. C., Orr F. W., Bastida E., Sauder D. N., Buchanan M. R. Differential effects of interleukin-1 and formylmethionylleucylphenylalanine on chemotaxis and human endothelium adhesivity for A549 tumor cells. Lab Invest. 1989 Jan;60(1):161–164. [PubMed] [Google Scholar]
- Leroyer V., Werner L., Shaughnessy S., Goddard G. J., Orr F. W. Chemiluminescence and oxygen radical generation by Walker carcinosarcoma cells following chemotactic stimulation. Cancer Res. 1987 Sep 15;47(18):4771–4775. [PubMed] [Google Scholar]
- Nakajima M., Welch D. R., Belloni P. N., Nicolson G. L. Degradation of basement membrane type IV collagen and lung subendothelial matrix by rat mammary adenocarcinoma cell clones of differing metastatic potentials. Cancer Res. 1987 Sep 15;47(18):4869–4876. [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Metastatic tumor cell attachment and invasion assay utilizing vascular endothelial cell monolayers. J Histochem Cytochem. 1982 Mar;30(3):214–220. doi: 10.1177/30.3.7061823. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Warner D. J. Effects of neutrophil-mediated pulmonary endothelial injury on the localization and metastasis of circulating Walker carcinosarcoma cells. Invasion Metastasis. 1987;7(3):183–196. [PubMed] [Google Scholar]
- Pryor W. A. Oxy-radicals and related species: their formation, lifetimes, and reactions. Annu Rev Physiol. 1986;48:657–667. doi: 10.1146/annurev.ph.48.030186.003301. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Pharmacological modulation of chemotactic factor-elicited release of granule-associated enzymes from human neutrophils. Effects of prostaglandins, nonsteroid anti-inflammatory agents and corticosteroids. Biochem Pharmacol. 1980 Sep 1;29(17):2389–2395. doi: 10.1016/0006-2952(80)90274-9. [DOI] [PubMed] [Google Scholar]
- Suttorp N., Toepfer W., Roka L. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol. 1986 Nov;251(5 Pt 1):C671–C680. doi: 10.1152/ajpcell.1986.251.5.C671. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Fantone J. C. Phorbol myristate acetate-induced adherence of Walker 256 carcinosarcoma cells. Cancer Res. 1982 Jan;42(1):190–197. [PubMed] [Google Scholar]
- Ward P. A., Sulavik M. C., Johnson K. J. Rat neutrophil activation and effects of lipoxygenase and cyclooxygenase inhibitors. Am J Pathol. 1984 Aug;116(2):223–233. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Till G. O., Kunkel R., Beauchamp C. Evidence for role of hydroxyl radical in complement and neutrophil-dependent tissue injury. J Clin Invest. 1983 Sep;72(3):789–801. doi: 10.1172/JCI111050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L., Orr F. W., Honn K. V. Interactions of cancer cells with the microvasculature during metastasis. FASEB J. 1988 Jan;2(1):12–21. doi: 10.1096/fasebj.2.1.3275560. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]



