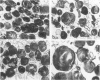
Abstract
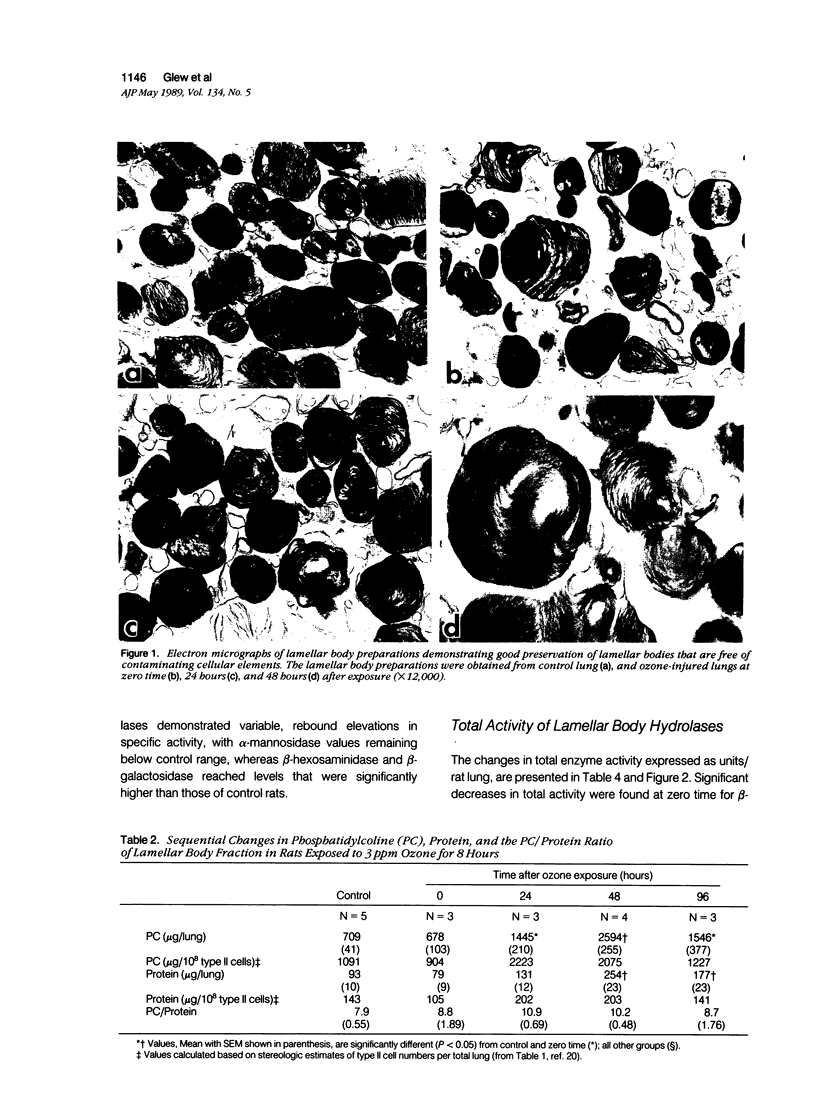
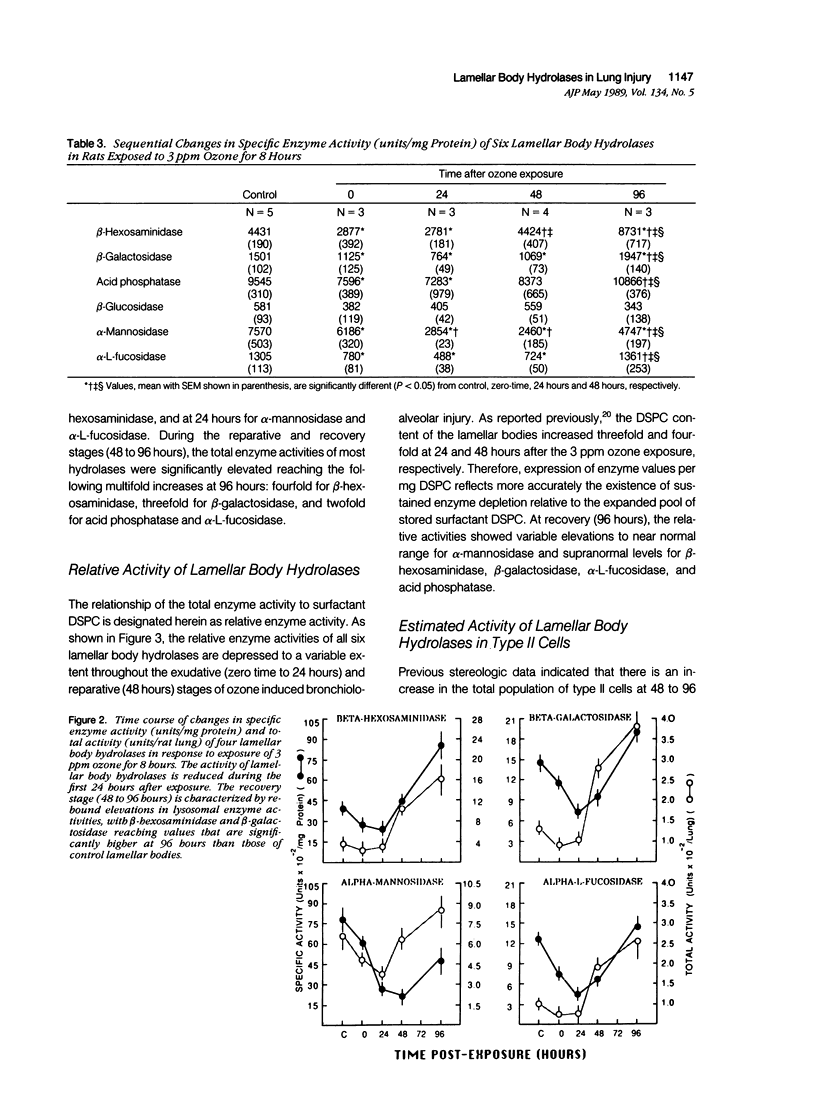
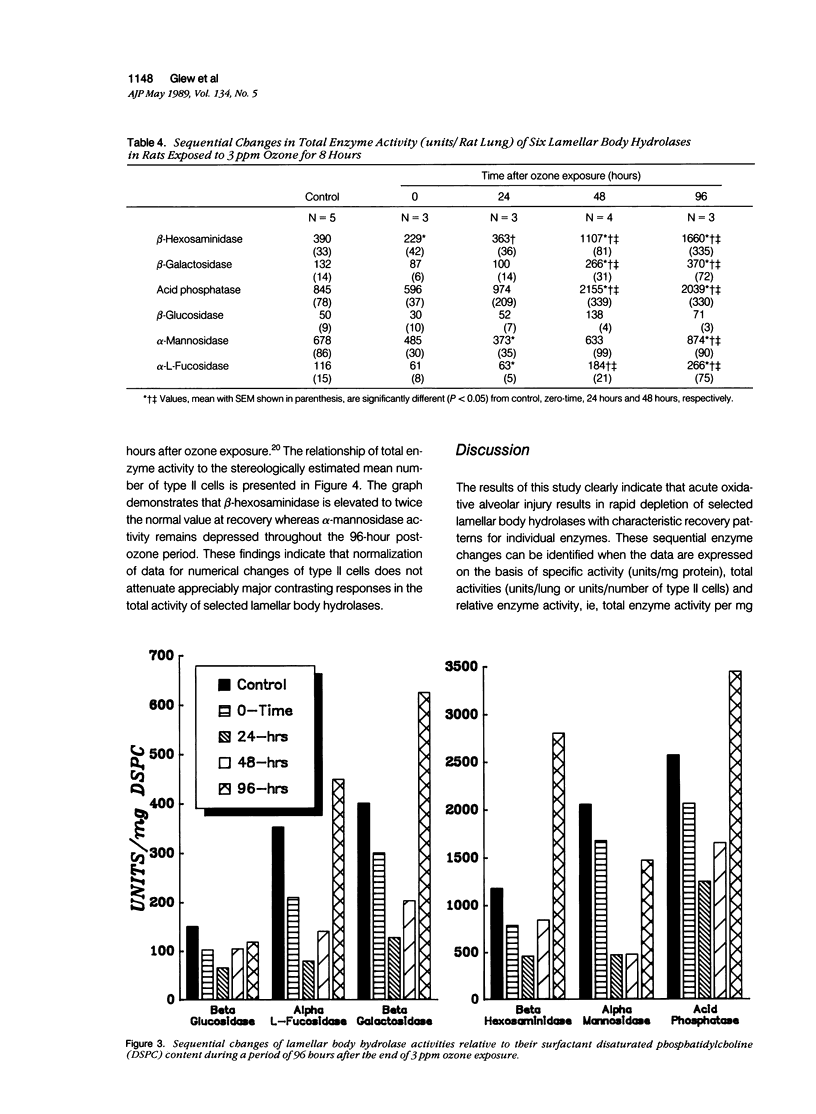
Lamellar body hydrolases in acutely damaged and regenerating type II cells were determined using an established rat model with well-defined stages of bronchiolo-alveolar injury and repair. Lamellar bodies were isolated from control and ozone-exposed (3.0 ppm for 8 hours) adult male rats by sucrose density gradient centrifugation and analyzed for their content of six different lysosomal hydrolases. Immediately after 3 ppm ozone exposure (zero-time) there was a significant decrease in specific enzyme activity (units/mg protein) of five lamellar body hydrolases and these activities remained depressed for at least 24 hours after exposure. In addition, total enzyme activity (units/lung) was reduced at zero-time for beta-hexosaminidase and at 24 hours postexposure for alpha-mannosidase and alpha-L-fucosidase. During the reparative and recovery stages (48 to 96 hours) the hydrolases demonstrated variable elevations in both specific activity and total activity (units/lung). Characteristically, beta-hexosaminidase and beta-galactosidase reached supranormal values at 96 hours, whereas alpha-mannosidase remained below normal levels through the recovery stage. Moreover, at 24 to 48 hours the lamellar body fraction demonstrated prominent enzyme depletion relative to the expanding pool of stored surfactant. It is concluded that acute ozone stress initiates the development of hydrolase deficiency within the lamellar bodies of injured and regenerating type II cells. This deficiency state is followed by asynchronous lamellar body hydrolase elevations that reflect distinct patterns of response rather than uniform return to normal condition. The lysosomal enzyme changes of lamellar bodies may be pathogenetically linked to the development of associated alterations in the storage and secretion of surfactant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALIS J. U., CONEN P. E. THE ROLE OF ALVEOLAR INCLUSION BODIES IN THE DEVELOPING LUNG. Lab Invest. 1964 Oct;13:1215–1229. [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Haller E. M., Shelley S. A., Montgomery M. R. Ozone-induced lamellar body responses in a rat model for alveolar injury and repair. Am J Pathol. 1988 Aug;132(2):330–344. [PMC free article] [PubMed] [Google Scholar]
- Balis J. U., Paterson J. F., Paciga J. E., Haller E. M., Shelley S. A. Distribution and subcellular localization of surfactant-associated glycoproteins in human lung. Lab Invest. 1985 Jun;52(6):657–669. [PubMed] [Google Scholar]
- Bienkowski R. S. Intracellular degradation of newly synthesized secretory proteins. Biochem J. 1983 Jul 15;214(1):1–10. doi: 10.1042/bj2140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K. R., Poduslo J. F. Lysosomal delivery of the major myelin glycoprotein in the absence of myelin assembly: posttranslational regulation of the level of expression by Schwann cells. J Cell Biol. 1987 Mar;104(3):661–669. doi: 10.1083/jcb.104.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander A., Johnson R. G., Reicherter J., Fisher A. B. Lung lamellar bodies maintain an acidic internal pH. J Biol Chem. 1986 May 5;261(13):6126–6131. [PubMed] [Google Scholar]
- DiAugustine R. P. Lung concentric laminar organelle. Hydrolase activity and compositional analysis. J Biol Chem. 1974 Jan 25;249(2):584–593. [PubMed] [Google Scholar]
- Duck-Chong C. G. The isolation of lamellar bodies and their membranous content from rat lung, lamb tracheal fluid and human amniotic fluid. Life Sci. 1978 Jun 12;22(22):2025–2030. doi: 10.1016/0024-3205(78)90549-0. [DOI] [PubMed] [Google Scholar]
- Golfischer S., Kikkawa Y., Hoffman L. The demonstration of acid hydrolase activities in the inclusion bodies of type II alveolar cells and other lysosomes in the rabbit lung. J Histochem Cytochem. 1968 Feb;16(2):102–109. doi: 10.1177/16.2.102. [DOI] [PubMed] [Google Scholar]
- Heath M. F., Jacobson W. Phospholipases A1 and A2 in lamellar inclusion bodies of the alveolar epithelium of rabbit lung. Biochim Biophys Acta. 1976 Sep 27;441(3):443–452. doi: 10.1016/0005-2760(76)90241-1. [DOI] [PubMed] [Google Scholar]
- Hook G. E. Extracellular hydrolases of the lung. Biochemistry. 1978 Feb 7;17(3):520–528. doi: 10.1021/bi00596a023. [DOI] [PubMed] [Google Scholar]
- Hook G. E., Gilmore L. B. Hydrolases of pulmonary lysosomes and lamellar bodies. J Biol Chem. 1982 Aug 10;257(15):9211–9220. [PubMed] [Google Scholar]
- Kuhn C., 3rd Cytochemistry of pulmonary alveolar epithelial cells. Am J Pathol. 1968 Nov;53(5):809–833. [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Tolbert N. E., Bieber L. L. Protein determination in membrane and lipoprotein samples: manual and automated procedures. Methods Enzymol. 1981;72:296–303. doi: 10.1016/s0076-6879(81)72018-4. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- Meban C. A cytochemical study of the granular pneumonocytes in hamster lung. J Anat. 1972 Feb;111(Pt 2):293–302. [PMC free article] [PubMed] [Google Scholar]
- Montgomery M. R., Anderson R. E., Mortenson G. A. A compact, versatile inhalation exposure chamber for small animal studies. Lab Anim Sci. 1976 Jun;26(3):461–464. [PubMed] [Google Scholar]
- Peters S. P., Lee R. E., Glew R. H. A microassay for Gaucher's disease. Clin Chim Acta. 1975 May 1;60(3):391–396. doi: 10.1016/0009-8981(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Post M., van Golde L. M. Metabolic and developmental aspects of the pulmonary surfactant system. Biochim Biophys Acta. 1988 Jun 9;947(2):249–286. doi: 10.1016/0304-4157(88)90011-1. [DOI] [PubMed] [Google Scholar]
- Randell S. H., Sannes P. L. Cytochemical localization and biochemical evaluation of a lysosomal serine protease in lung: dipeptidyl peptidase II in the normal rat. J Histochem Cytochem. 1985 Jul;33(7):677–686. doi: 10.1177/33.7.3924993. [DOI] [PubMed] [Google Scholar]
- Rooney S. A. The surfactant system and lung phospholipid biochemistry. Am Rev Respir Dis. 1985 Mar;131(3):439–460. doi: 10.1164/arrd.1985.131.3.439. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Clements J. A. Metabolism and turnover of lung surfactant. Am Rev Respir Dis. 1987 Aug;136(2):426–444. doi: 10.1164/ajrccm/136.2.426. [DOI] [PubMed] [Google Scholar]
- de Vries A. C., Schram A. W., Tager J. M., Batenburg J. J., van Golde L. M. A specific acid alpha-glucosidase in lamellar bodies of the human lung. Biochim Biophys Acta. 1985 Dec 4;837(3):230–238. doi: 10.1016/0005-2760(85)90046-3. [DOI] [PubMed] [Google Scholar]
- de Vries A. C., Schram A. W., van den Berg M., Tager J. M., Batenburg J. J., van Golde L. M. An improved procedure for the isolation of lamellar bodies from human lung. Lamellar bodies free of lysosomes contain a spectrum of lysosomal-type hydrolases. Biochim Biophys Acta. 1987 Dec 14;922(3):259–269. doi: 10.1016/0005-2760(87)90048-8. [DOI] [PubMed] [Google Scholar]