Abstract
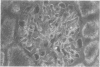
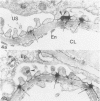
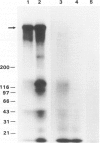
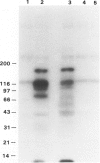
To investigate the role of glomerular epithelial cell (GEC) membrane proteins in the in situ formation of subepithelial immune deposits, the authors raised a rabbit antiserum against GEC that had been grown in culture (anti-GEC). By indirect immunofluorescence (IF) on normal rat kidney, anti-GEC stained proximal tubular brush border (BB). After intravenous injection into animals, granular glomerular capillary wall staining for IgG was present by IE and subepithelial immune deposits were identified by standard transmission and immunoelectron microscopy. Using the latter technique, injected anti-GEC IgG was identified beneath slit diaphragms and in endocytic-coated pits and intracellular vesicles of podocytes. Anti-GEC immunoprecipitated gp330 and two other proteins from radiolabeled BB. These proteins also were identified by sheep anti-rat Fx1A, the antiserum responsible for passive Heymann nephritis. Anti-GEC and anti-Fx1A also immunoprecipitated five identical proteins from surface-labeled GEC. Biosynthetically-labeled but not surface-labeled GEC contained immunoprecipitable gp330. Thus, injection into rats of antibodies raised against cultured GEC can produce subepithelial immune deposits, a disease process classically induced by antibodies to BB or its purified components. In addition to gp330, GEC and BB share other antigenic determinants that may contribute to the formation of these immune deposits.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Caulfield J. P. Distribution of laminin within rat and mouse renal, splenic, intestinal, and hepatic basement membranes identified after the intravenous injection of heterologous antilaminin IgG. Lab Invest. 1985 Feb;52(2):169–181. [PubMed] [Google Scholar]
- Bertani T., Nolin L., Foidart J., Vandewalle A., Verroust P. The effect of puromycin on subepithelial deposits induced by antibodies directed against tubular antigens: a quantitative study. Eur J Clin Invest. 1979 Dec;9(6):465–472. doi: 10.1111/j.1365-2362.1979.tb00914.x. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Baird L. G., Collins A. B., Kamata K., Bradford D., Erikson M. E., McCluskey R. T. Studies with monoclonal antibodies against brush border antigens in Heymann nephritis. Lab Invest. 1985 Oct;53(4):421–432. [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Brentjens J. R., Noble B., Kerjaschki D., Malavasi F., Roholt O. A., Farquhar M. G., Andres G. Antibody-induced redistribution of Heymann antigen on the surface of cultured glomerular visceral epithelial cells: possible role in the pathogenesis of Heymann glomerulonephritis. J Immunol. 1985 Oct;135(4):2409–2416. [PubMed] [Google Scholar]
- Camussi G., Salvidio G., Biesecker G., Brentjens J., Andres G. Heymann antibodies induce complement-dependent injury of rat glomerular visceral epithelial cells. J Immunol. 1987 Nov 1;139(9):2906–2914. [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky A. V., Quigg R. J., Badalamenti J., Salant D. J. Anti-Fx1A induces association of Heymann nephritis antigens with microfilaments of cultured glomerular visceral epithelial cells. Am J Pathol. 1987 Nov;129(2):373–384. [PMC free article] [PubMed] [Google Scholar]
- Feenstra K., van den Lee R., Greben H. A., Arends A., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. I. The natural history: a histologic and immunohistologic study at the light microscopic and the ultrastructural level. Lab Invest. 1975 Feb;32(2):235–242. [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. Externally disposed plasma membrane proteins. I. Enzymatic iodination of mouse L cells. J Cell Biol. 1975 Feb;64(2):438–460. doi: 10.1083/jcb.64.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K., Baird L. G., Erikson M. E., Collins A. B., McCluskey R. T. Characterization of antigens and antibody specificities involved in Heymann nephritis. J Immunol. 1985 Oct;135(4):2400–2408. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krangel M. S. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987 Apr 1;165(4):1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg J. I., Karnovsky M. J. Glomerular cells in culture. Kidney Int. 1983 Mar;23(3):439–447. doi: 10.1038/ki.1983.40. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makker S. P., Singh A. K. Characterization of the antigen (gp600) of Heymann nephritis. Lab Invest. 1984 Mar;50(3):287–293. [PubMed] [Google Scholar]
- Mendrick D. L., Rennke H. G. Epitope specific induction of proteinuria by monoclonal antibodies. Kidney Int. 1988 Apr;33(4):831–842. doi: 10.1038/ki.1988.74. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Natori Y., Hayakawa I., Shibata S. Identification of gp108, a pathogenic antigen of passive Heymann nephritis, as dipeptidyl peptidase IV. Clin Exp Immunol. 1987 Nov;70(2):434–439. [PMC free article] [PubMed] [Google Scholar]
- Natori Y., Hayakawa I., Shibata S. Passive Heymann nephritis with acute and severe proteinuria induced by heterologous antibody against renal tubular brush border glycoprotein gp108. Lab Invest. 1986 Jul;55(1):63–70. [PubMed] [Google Scholar]
- Quigg R. J., Cybulsky A. V., Jacobs J. B., Salant D. J. Anti-Fx1A produces complement-dependent cytotoxicity of glomerular epithelial cells. Kidney Int. 1988 Jul;34(1):43–52. doi: 10.1038/ki.1988.143. [DOI] [PubMed] [Google Scholar]
- Quigg R. J., Nicholson-Weller A., Cybulsky A. V., Badalamenti J., Salant D. J. Decay accelerating factor regulates complement activation on glomerular epithelial cells. J Immunol. 1989 Feb 1;142(3):877–882. [PubMed] [Google Scholar]
- Ronco P., Allegri L., Melcion C., Pirotsky E., Appay M. D., Bariety J., Pontillon F., Verroust P. A monoclonal antibody to brush border and passive Heymann nephritis. Clin Exp Immunol. 1984 Feb;55(2):319–332. [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Belok S., Madaio M. P., Couser W. G. A new role for complement in experimental membranous nephropathy in rats. J Clin Invest. 1980 Dec;66(6):1339–1350. doi: 10.1172/JCI109987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salant D. J., Darby C., Couser W. G. Experimental membranous glomerulonephritis in rats. Quantitative studies of glomerular immune deposit formation in isolated glomeruli and whole animals. J Clin Invest. 1980 Jul;66(1):71–81. doi: 10.1172/JCI109837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schwartz A. L. Receptor-mediated endocytosis. J Clin Invest. 1986 Mar;77(3):657–662. doi: 10.1172/JCI112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker G. E., Striker L. J. Glomerular cell culture. Lab Invest. 1985 Aug;53(2):122–131. [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]
- Verroust P., Ronco P., Chatelet F. Antigenic targets in membranous glomerulonephritis. Springer Semin Immunopathol. 1987;9(4):341–358. doi: 10.1007/BF00197213. [DOI] [PubMed] [Google Scholar]