Abstract
Griffonia simplicifolia agglutinin (GSA) is a valuable histochemical tool in the identification of endothelium. In this study GSA labeled with fluorescein isothiocyanate (GSA-FITC) was used to purify cultures of murine cerebral microvascular endothelium. Cultures were stained with GSA-FITC, then sorted using a fluorescence-activated cell sorter (FACS). GSA-positive endothelial cells were collected, re-cultured, and subsequently re-analyzed by FACS using GSA-FITC. Cultures that initially contained 80 +/- 3 to 89 +/- 3% (X +/- SE) GSA-positive cells were purified to 98 +/- 1% positivity. Immunohistochemistry with an anti-muscle-action antibody confirmed that FACS sorting of GSA-FITC-stained cells effectively removed contaminating smooth muscle cells from endothelial cell cultures. Viability, proliferation, and prostaglandin production of the cells was unaltered by lectin staining and FACS sorting. Thus, GSA-FITC can be used in conjunction with flow cytometry to enhance the purity of murine endothelial cell cultures without adversely affecting cell viability, growth, or metabolism.
Full text
PDF

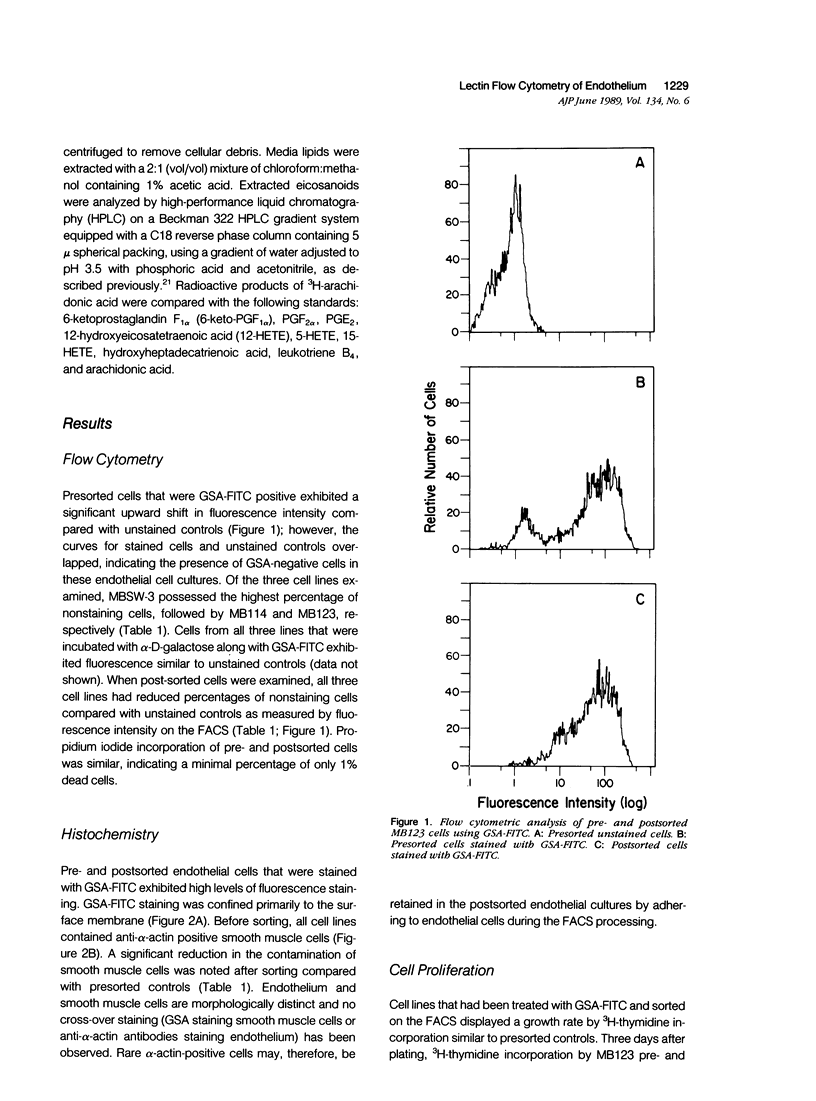
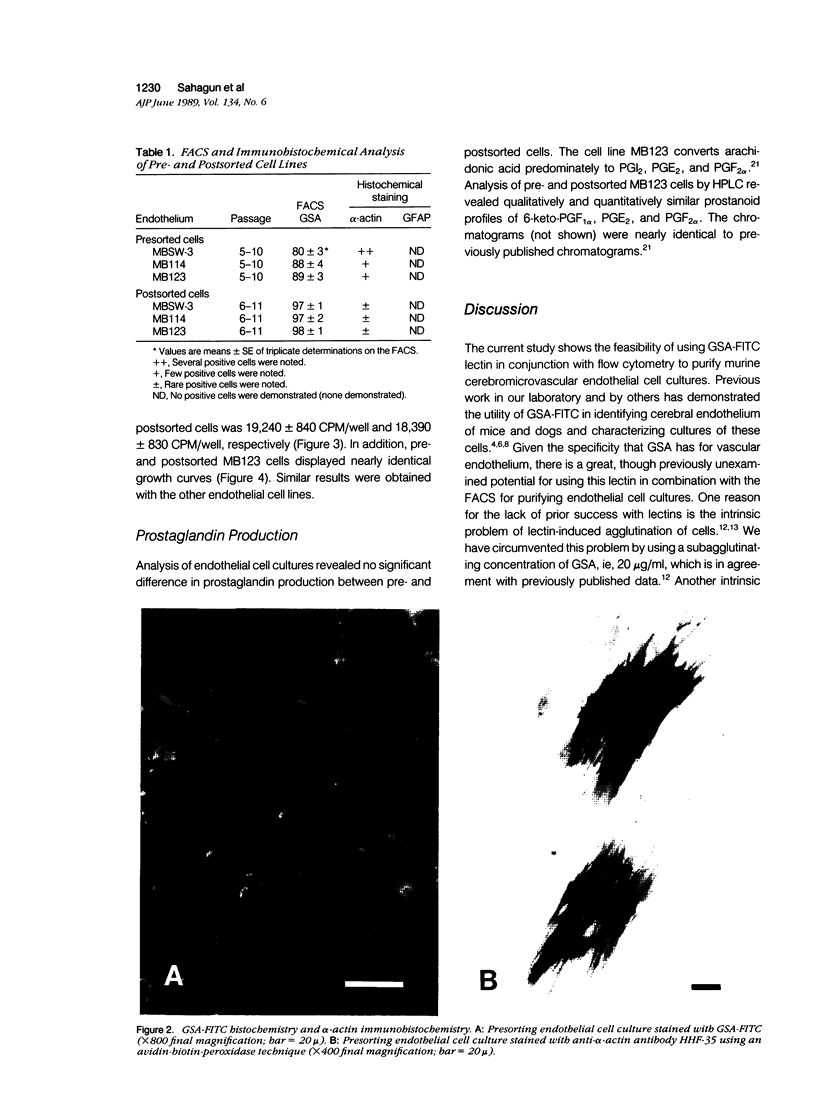
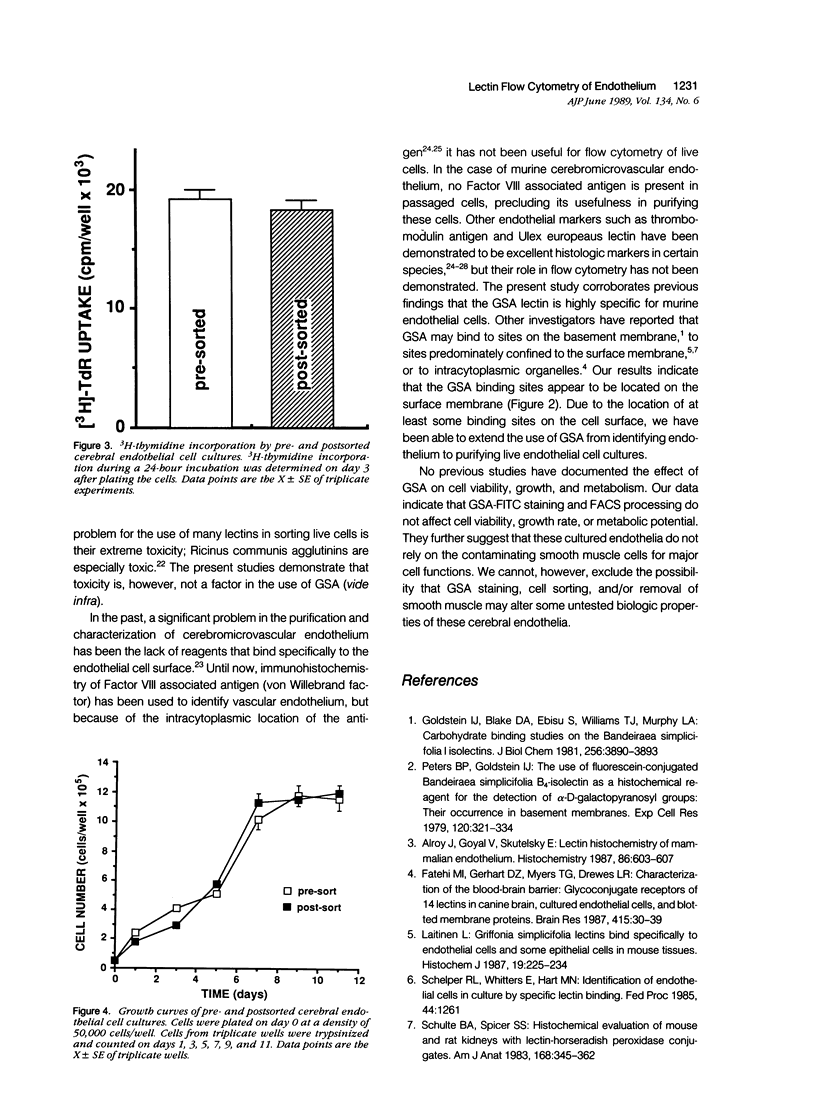

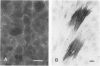
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alroy J., Goyal V., Skutelsky E. Lectin histochemistry of mammalian endothelium. Histochemistry. 1987;86(6):603–607. doi: 10.1007/BF00489554. [DOI] [PubMed] [Google Scholar]
- Ault K. A. Clinical applications of fluorescence-activated cell sorting techniques. Diagn Immunol. 1983;1(1):2–10. [PubMed] [Google Scholar]
- Bowman P. D., Betz A. L., Ar D., Wolinsky J. S., Penney J. B., Shivers R. R., Goldstein G. W. Primary culture of capillary endothelium from rat brain. In Vitro. 1981 Apr;17(4):353–362. doi: 10.1007/BF02618147. [DOI] [PubMed] [Google Scholar]
- DeBault L. E., Esmon N. L., Smith G. P., Esmon C. T. Localization of thrombomodulin antigen in rabbit endothelial cells in culture. An immunofluorescence and immunoelectron microscope study. Lab Invest. 1986 Feb;54(2):179–187. [PubMed] [Google Scholar]
- DeBault L. E., Henriquez E., Hart M. N., Cancilla P. A. Cerebral microvessels and derived cells in tissue culture: II. Establishment, identification, and preliminary characterization of an endothelial cell line. In Vitro. 1981 Jun;17(6):480–494. doi: 10.1007/BF02633509. [DOI] [PubMed] [Google Scholar]
- DeBault L. E., Kahn L. E., Frommes S. P., Cancilla P. A. Cerebral microvessels and derived cells in tissue culture: isolation and preliminary characterization. In Vitro. 1979 Jul;15(7):473–487. doi: 10.1007/BF02618149. [DOI] [PubMed] [Google Scholar]
- Doolittle M., Bohman R., Durstenfeld A., Cascarano J. Identification and characterization of liver nonparenchymal cells by flow cytometry. Hepatology. 1987 Jul-Aug;7(4):696–703. doi: 10.1002/hep.1840070413. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Fatehi M. I., Gerhart D. Z., Myers T. G., Drewes L. R. Characterization of the blood-brain barrier: glycoconjugate receptors of 14 lectins in canine brain, cultured endothelial cells, and blotted membrane proteins. Brain Res. 1987 Jul 7;415(1):30–39. doi: 10.1016/0006-8993(87)90266-6. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Blake D. A., Ebisu S., Williams T. J., Murphy L. A. Carbohydrate binding studies on the Bandeiraea simplicifolia I isolectins. Lectins which are mono-, di-, tri-, and tetravalent for N-acetyl-D-galactosamine. J Biol Chem. 1981 Apr 25;256(8):3890–3893. [PubMed] [Google Scholar]
- Hamburger A. W., Reid Y. A., Pelle B. A., Breth L. A., Beg N., Ryan U., Cines D. B. Isolation and characterization of monoclonal antibodies reactive with endothelial cells. Tissue Cell. 1985;17(4):451–459. doi: 10.1016/0040-8166(85)90024-2. [DOI] [PubMed] [Google Scholar]
- Imai Y., Oguchi T., Nakano T., Osawa T. Separation of mouse T cell subsets by a fluorescent activated cell sorter using fluorescence-labeled peanut agglutinin. Immunol Commun. 1979;8(5-6):495–503. doi: 10.3109/08820137909063248. [DOI] [PubMed] [Google Scholar]
- Laitinen L. Griffonia simplicifolia lectins bind specifically to endothelial cells and some epithelial cells in mouse tissues. Histochem J. 1987 Apr;19(4):225–234. doi: 10.1007/BF01680633. [DOI] [PubMed] [Google Scholar]
- Malin-Berdel J., Valet G., Thiel E., Forrester J. A., Gürtler L. Flow cytometric analysis of the binding of eleven lectins to human T- and B-cells and to human T- and B-cell lines. Cytometry. 1984 Mar;5(2):204–209. doi: 10.1002/cyto.990050215. [DOI] [PubMed] [Google Scholar]
- McCoy J. P., Jr, Shibuya N., Riedy M. C., Goldstein I. J. Griffonia simplicifolia I isolectin as a functionally monovalent probe for use in flow cytometry. Cytometry. 1986 Mar;7(2):142–146. doi: 10.1002/cyto.990070204. [DOI] [PubMed] [Google Scholar]
- Moore S. A., Spector A. A., Hart M. N. Eicosanoid metabolism in cerebromicrovascular endothelium. Am J Physiol. 1988 Jan;254(1 Pt 1):C37–C44. doi: 10.1152/ajpcell.1988.254.1.C37. [DOI] [PubMed] [Google Scholar]
- Morin O., Patry P., Lafleur L. Heterogeneity of endothelial cells of adult rat liver as resolved by sedimentation velocity and flow cytometry. J Cell Physiol. 1984 Jun;119(3):327–334. doi: 10.1002/jcp.1041190311. [DOI] [PubMed] [Google Scholar]
- Peters B. P., Goldstein I. J. The use of fluorescein-conjugated Bandeiraea simplicifolia B4-isolectin as a histochemical reagent for the detection of alpha-D-galactopyranosyl groups. Their occurrence in basement membranes. Exp Cell Res. 1979 May;120(2):321–334. doi: 10.1016/0014-4827(79)90392-6. [DOI] [PubMed] [Google Scholar]
- Preobrazhensky S. N., Antonov A. S., Perova N. V., Sherbakova I. A., Samokhin G. P., Gerasimova E. N., Repin V. S., Smirnov V. N. Flow cytofluorometric study of lipoprotein interactions with cultured endothelial cells. Cytometry. 1982 Nov;3(3):172–176. doi: 10.1002/cyto.990030306. [DOI] [PubMed] [Google Scholar]
- Schulte B. A., Spicer S. S. Histochemical evaluation of mouse and rat kidneys with lectin-horseradish peroxidase conjugates. Am J Anat. 1983 Nov;168(3):345–362. doi: 10.1002/aja.1001680308. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Olmsted J. B., Marder V. J. Immunolocalization of von Willebrand protein in Weibel-Palade bodies of human endothelial cells. J Cell Biol. 1982 Oct;95(1):355–360. doi: 10.1083/jcb.95.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A. Ulex europeus I--peroxidase as a marker of vascular endothelium: its application in routine histopathology. J Pathol. 1985 Jun;146(2):123–127. doi: 10.1002/path.1711460207. [DOI] [PubMed] [Google Scholar]