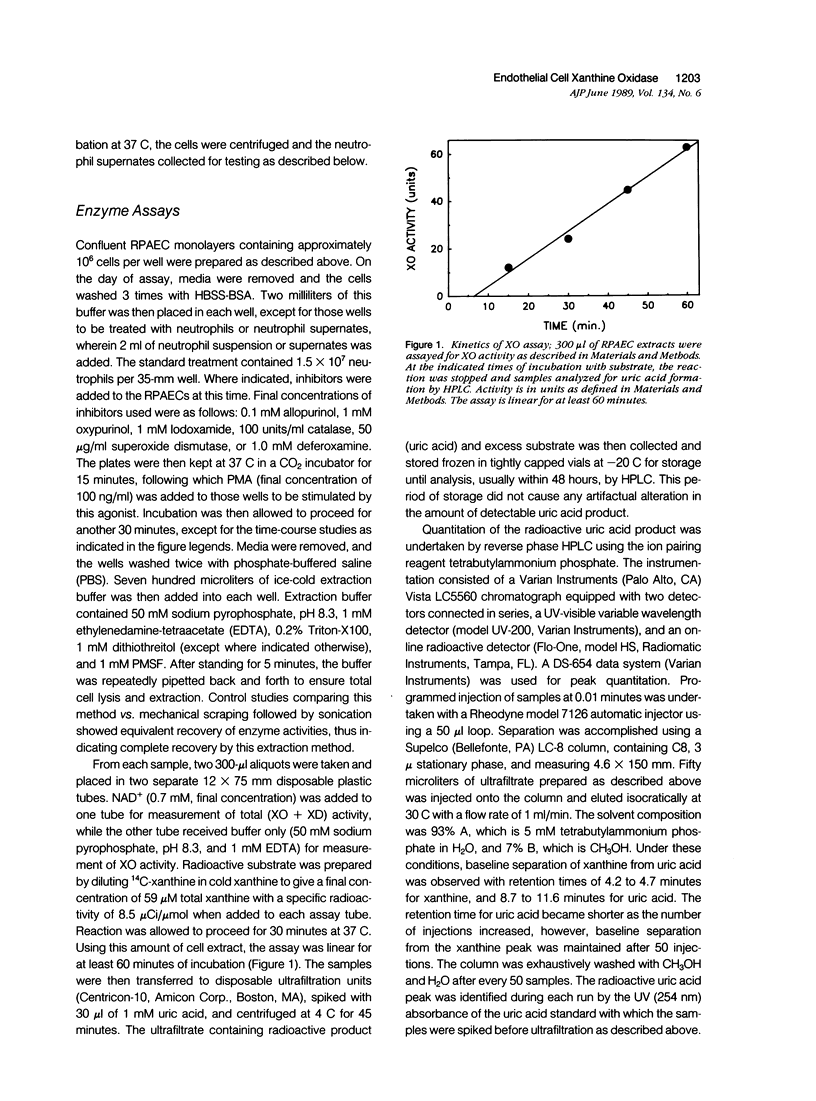
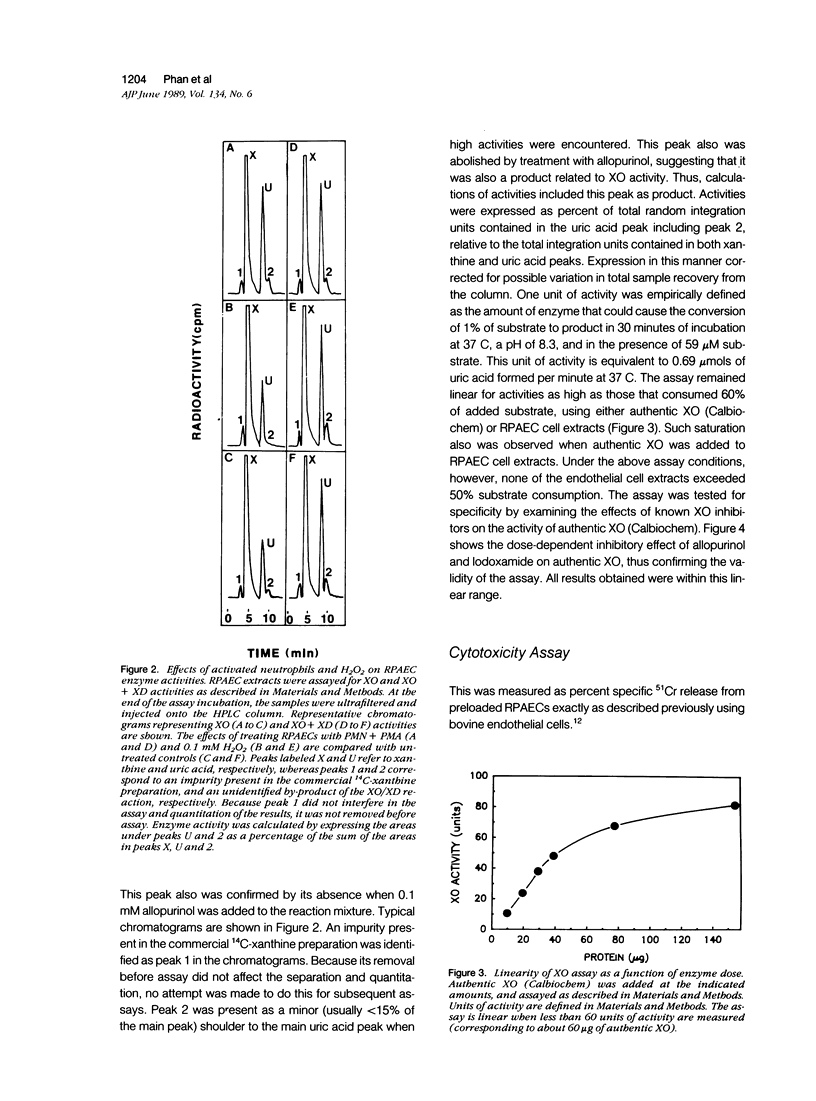
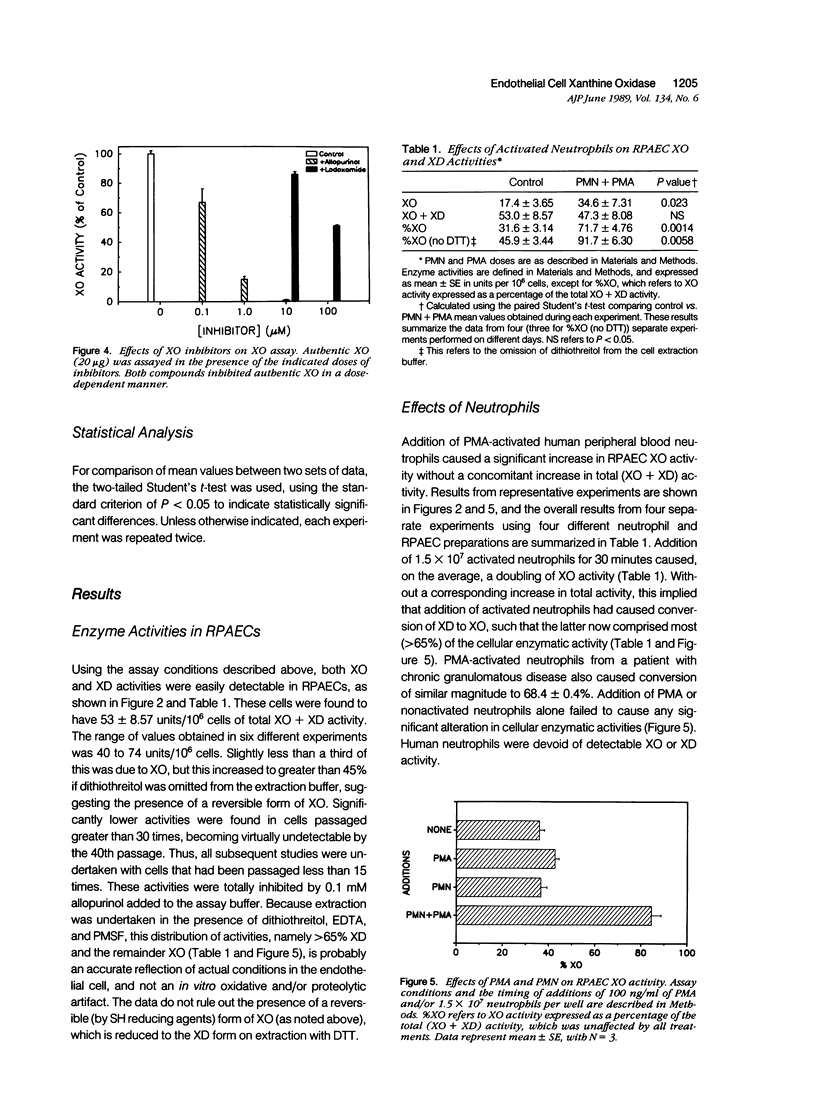
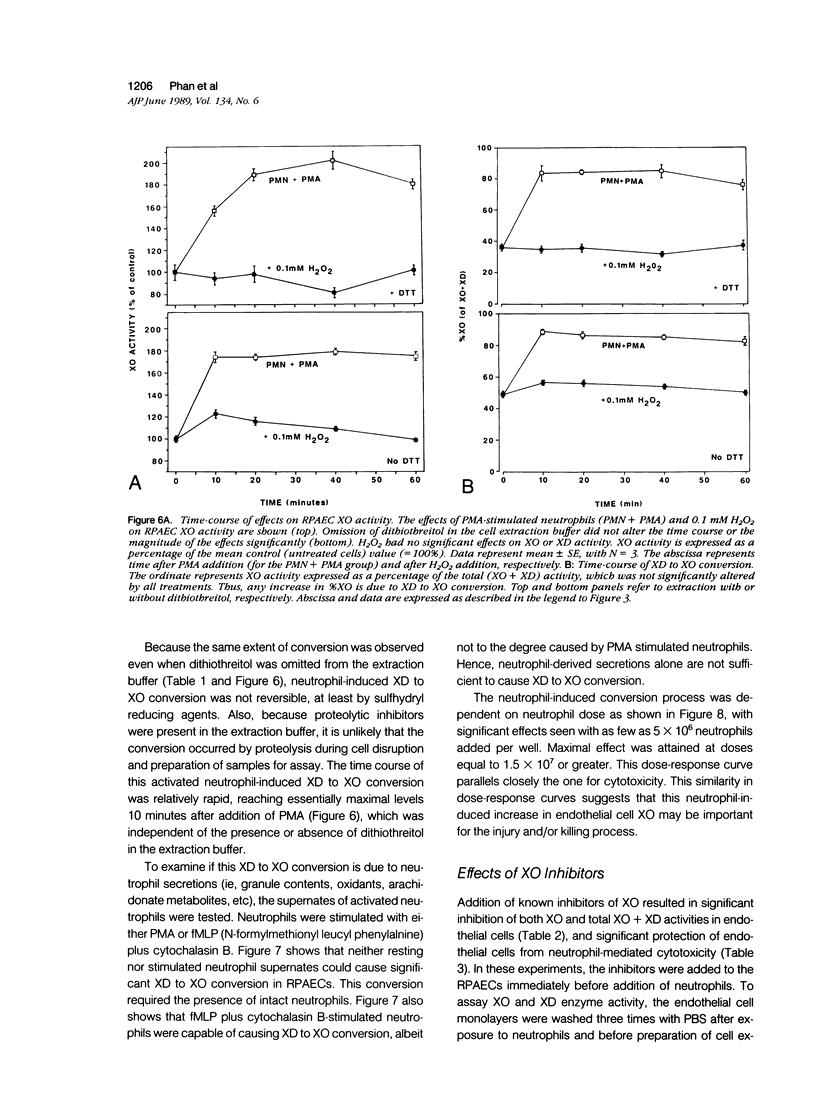
Abstract
The possibility that endothelial cell-derived oxidants could contribute to neutrophil-mediated endothelial cell injury and cytotoxicity has been a subject of speculation. Rat pulmonary artery endothelial cells (RPAECs) were examined for the presence of xanthine oxidase (XO) activity, a well-known source of O2-. Using a sensitive assay based on measurements of radioactive xanthine conversion to uric acid by high performance liquid chromatography (HPLC), RPAEC extracts were found to contain both XO and xanthine dehydrogenase (XD) activities. Extracts from early passage cells have 55.3 +/- 11.7 (mean +/- SE) units/10(6) cells of total (XO + XD) activity, one unit of activity being defined as the conversion of 1% of substrate to product in 30 minutes of incubation. XO comprised 31.6 +/- 3.1% of this total activity. Addition of human neutrophils stimulated with phorbol myristate acetate (PMA) caused a rapid and dose-dependent increase in RPAEC XO activity from 31.6 +/- 3.1% to 71.7 +/- 4.8% of total without altering total (XO + XD) activity. The neutrophil dose-response curve for increase in XO paralleled closely the curve for neutrophil-mediated RPAEC cytotoxicity. The basal XO and XD activities and the neutrophil-induced increase in XO activity were inhibited by treating RPAECs with allopurinol, oxypurinol, and lodoxamide, which also inhibited cytotoxicity, but not by catalase, superoxide dismutase, or deferoxamine. Addition of H2O2 failed to cause an increase in RPAEC XO activity or XD to XO conversion. The results suggest that during neutrophil-mediated injury, rapid conversion of RPAEC XD to XO occurs, resulting in increased XO, catalyzed endogenous oxidant production, which may contribute to the oxidant burden in the killing mechanism initiated by activated neutrophils. Although the mechanism for conversion of XD to XO is uncertain, it appears that neutrophil-derived H2O2 is not sufficient to cause this phenomenon. Furthermore, neither O2- nor chelatable iron is required for neutrophil-induced XD to XO conversion. Supernatant fluids from activated neutrophils failed to induce XD to XO conversion in RPAECs. This in vitro system provides an opportunity to define the cellular and molecular mechanisms underlying the in vivo phenomenon of XD to XO conversion associated with ischemic/reperfusion or inflammatory tissue injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli M. G., Lorenzoni E., Stripe F. Milk xanthine oxidase type D (dehydrogenase) and type O (oxidase). Purification, interconversion and some properties. Biochem J. 1973 Feb;131(2):191–198. doi: 10.1042/bj1310191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. A mechanism for the production of ethylene from methional. The generation of the hydroxyl radical by xanthine oxidase. J Biol Chem. 1970 Sep 25;245(18):4641–4646. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Fiers W., Cotran R. S., Gimbrone M. A., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4533–4537. doi: 10.1073/pnas.83.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B. Interactions of granulocytes with the lungs. Circ Res. 1984 Jun;54(6):623–635. doi: 10.1161/01.res.54.6.623. [DOI] [PubMed] [Google Scholar]
- Broudy V. C., Kaushansky K., Segal G. M., Harlan J. M., Adamson J. W. Tumor necrosis factor type alpha stimulates human endothelial cells to produce granulocyte/macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7467–7471. doi: 10.1073/pnas.83.19.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engerson T. D., McKelvey T. G., Rhyne D. B., Boggio E. B., Snyder S. J., Jones H. P. Conversion of xanthine dehydrogenase to oxidase in ischemic rat tissues. J Clin Invest. 1987 Jun;79(6):1564–1570. doi: 10.1172/JCI112990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon D. E., Varani J., Phan S. H., Ward J. H., Kaplan J., Till G. O., Simon R. H., Ryan U. S., Ward P. A. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987 Jul;57(1):37–44. [PubMed] [Google Scholar]
- Granger D. N., McCord J. M., Parks D. A., Hollwarth M. E. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology. 1986 Jan;90(1):80–84. doi: 10.1016/0016-5085(86)90078-8. [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Hajjar D. P., Silverstein R. L., Nachman R. L. Tumor necrosis factor-mediated release of platelet-derived growth factor from cultured endothelial cells. J Exp Med. 1987 Jul 1;166(1):235–245. doi: 10.1084/jem.166.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearse D. J., Manning A. S., Downey J. M., Yellon D. M. Xanthine oxidase: a critical mediator of myocardial injury during ischemia and reperfusion? Acta Physiol Scand Suppl. 1986;548:65–78. [PubMed] [Google Scholar]
- Jarasch E. D., Grund C., Bruder G., Heid H. W., Keenan T. W., Franke W. W. Localization of xanthine oxidase in mammary-gland epithelium and capillary endothelium. Cell. 1981 Jul;25(1):67–82. doi: 10.1016/0092-8674(81)90232-4. [DOI] [PubMed] [Google Scholar]
- Korthuis R. J., Granger D. N., Townsley M. I., Taylor A. E. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res. 1985 Oct;57(4):599–609. doi: 10.1161/01.res.57.4.599. [DOI] [PubMed] [Google Scholar]
- Martin W. J., 2nd Neutrophils kill pulmonary endothelial cells by a hydrogen-peroxide-dependent pathway. An in vitro model of neutrophil-mediated lung injury. Am Rev Respir Dis. 1984 Aug;130(2):209–213. doi: 10.1164/arrd.1984.130.2.209. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McIntyre T. M., Zimmerman G. A., Prescott S. M. Leukotrienes C4 and D4 stimulate human endothelial cells to synthesize platelet-activating factor and bind neutrophils. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2204–2208. doi: 10.1073/pnas.83.7.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Ratych R. E., Bulkley G. B. Free-radical-mediated postischemic reperfusion injury in the kidney. J Free Radic Biol Med. 1986;2(5-6):311–319. doi: 10.1016/s0748-5514(86)80030-7. [DOI] [PubMed] [Google Scholar]
- Ratych R. E., Chuknyiska R. S., Bulkley G. B. The primary localization of free radical generation after anoxia/reoxygenation in isolated endothelial cells. Surgery. 1987 Aug;102(2):122–131. [PubMed] [Google Scholar]
- Rodell T. C., Cheronis J. C., Ohnemus C. L., Piermattei D. J., Repine J. E. Xanthine oxidase mediates elastase-induced injury to isolated lungs and endothelium. J Appl Physiol (1985) 1987 Nov;63(5):2159–2163. doi: 10.1152/jappl.1987.63.5.2159. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Clements E., Habliston D., Ryan J. W. Isolation and culture of pulmonary artery endothelial cells. Tissue Cell. 1978;10(3):535–554. doi: 10.1016/s0040-8166(16)30347-0. [DOI] [PubMed] [Google Scholar]
- Ryan U. S. Isolation and culture of pulmonary endothelial cells. Environ Health Perspect. 1984 Jun;56:103–114. doi: 10.1289/ehp.8456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., White L. A., Lopez M., Ryan J. W. Use of microcarriers to isolate and culture pulmonary microvascular endothelium. Tissue Cell. 1982;14(3):597–606. doi: 10.1016/0040-8166(82)90050-7. [DOI] [PubMed] [Google Scholar]
- Schaffer S. W., Roy R. S., McMcord J. M. Possible role for calmodulin in calcium paradox-induced heart failure. Eur Heart J. 1983 Dec;4 (Suppl H):81–87. doi: 10.1093/eurheartj/4.suppl_h.81. [DOI] [PubMed] [Google Scholar]
- Schoutsen B., De Jong J. W., Harmsen E., De Tombe P. P., Achterberg P. W. Myocardial xanthine oxidase/dehydrogenase. Biochim Biophys Acta. 1983 Jul 14;762(4):519–524. doi: 10.1016/0167-4889(83)90055-1. [DOI] [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Waud W. R., Rajagopalan K. V. Purification and properties of the NAD+-dependent (type D) and O2-dependent (type O) forms of rat liver xanthine dehydrogenase. Arch Biochem Biophys. 1976 Feb;172(2):354–364. doi: 10.1016/0003-9861(76)90087-4. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Sutton H. C. Iron and xanthine oxidase catalyze formation of an oxidant species distinguishable from OH.: comparison with the Haber-Weiss reaction. Arch Biochem Biophys. 1986 Jan;244(1):27–34. doi: 10.1016/0003-9861(86)90090-1. [DOI] [PubMed] [Google Scholar]


