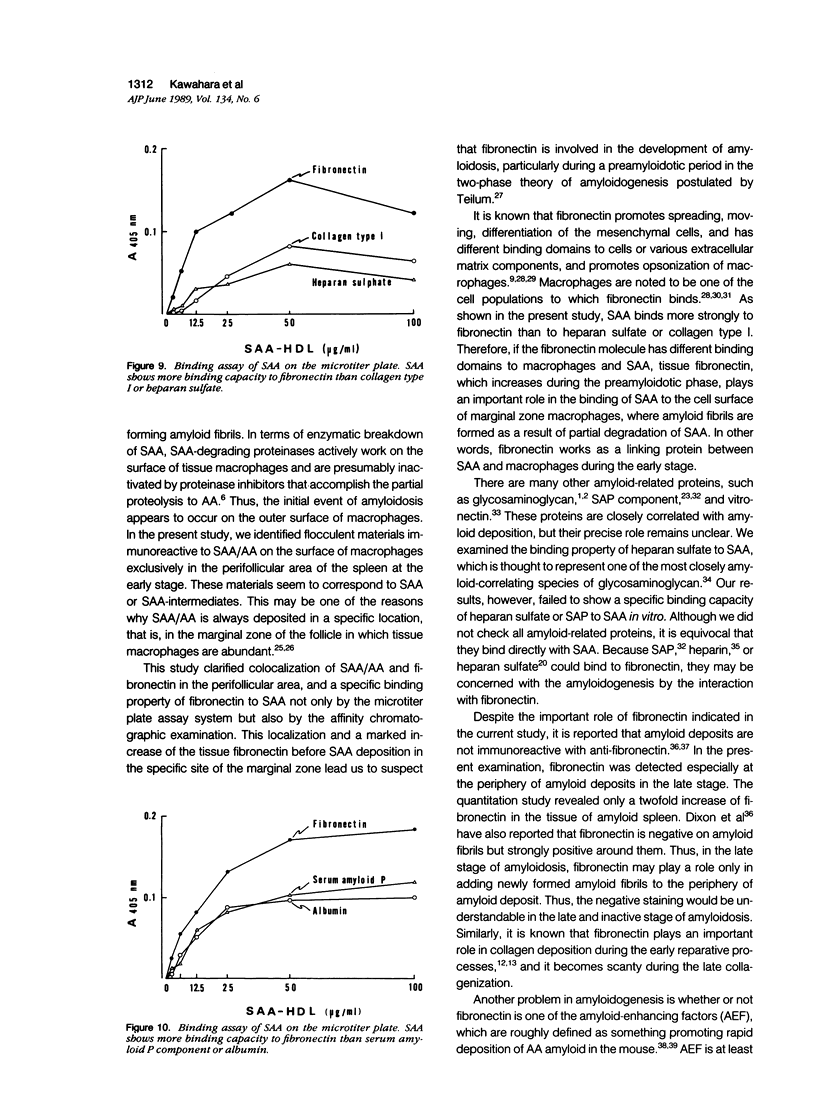
Abstract
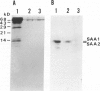
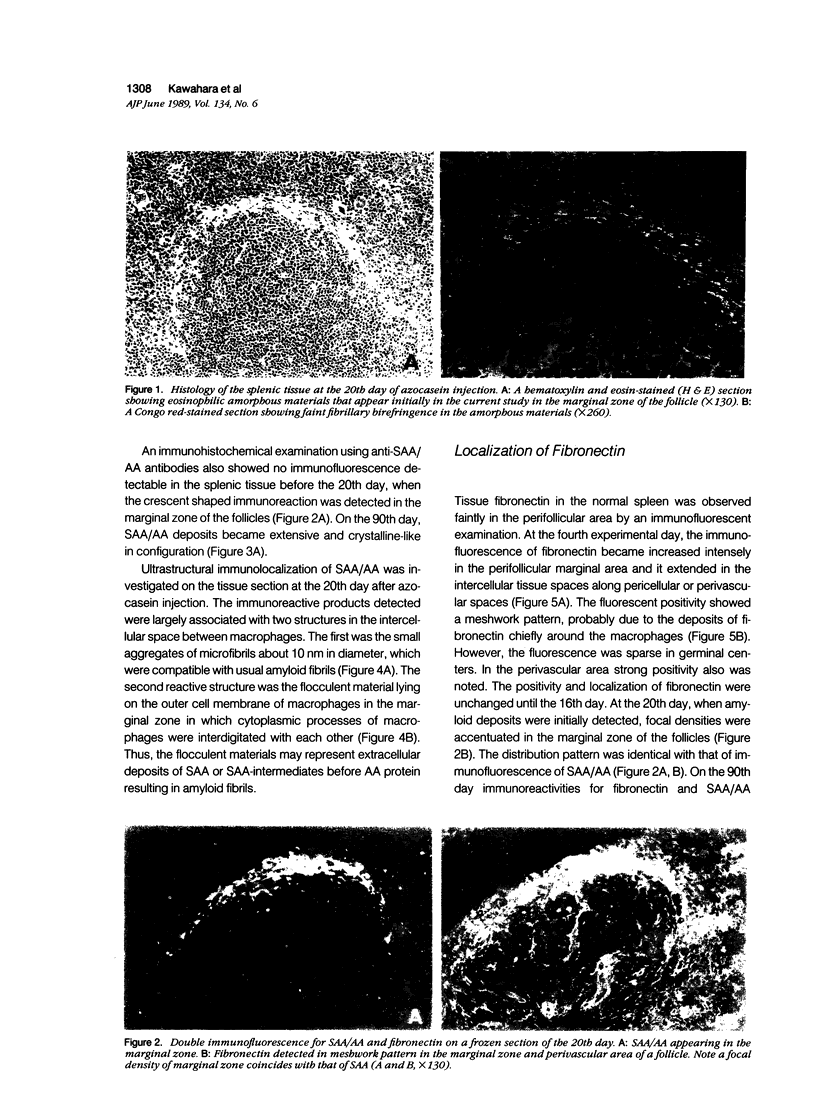
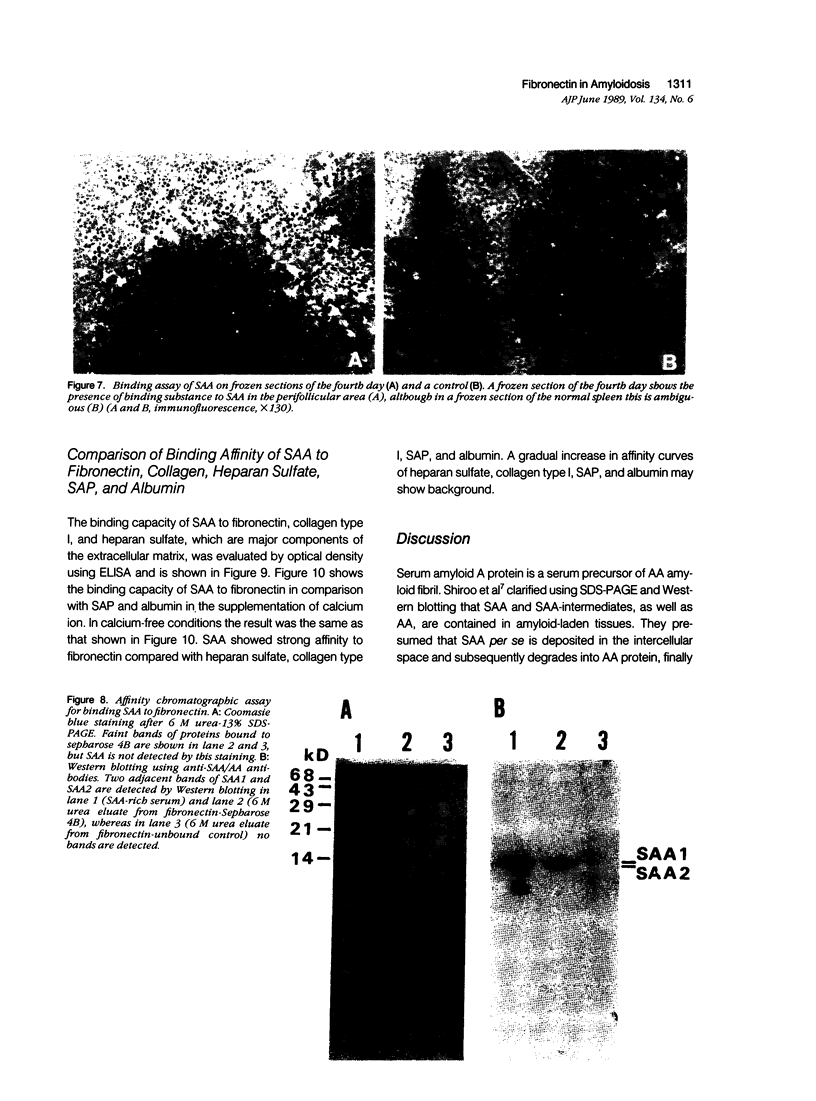
Azocasein-induced amyloid A (AA) amyloidosis in CBA/K1Jms mice was investigated to elucidate a preference of serum amyloid A (SAA) deposition in the spleen. By indirect immunofluorescence using anti-SAA/AA antibodies the initial deposition of SAA/AA was recognized in the marginal zone of spleen at 20 days after azocasein injection. Indirect immunofluorescence using anti-fibronectin antibodies also showed meshwork positivity in the corresponding area more intensely than that in controls. Immunoelectron microscopy using anti-SAA/AA revealed the presence of positively stained flocculent materials on cell surfaces of macrophages in the marginal area in addition to amyloid fibril. The tissue fibronectin rapidly increased in the spleen and maintained 10 times more than that of controls until the 20th day. Binding assay of SAA on frozen sections revealed the presence of SAA-binding substances in the perifollicular area. Affinity chromatographic assay showed fibronectin have a binding capacity to SAA1 and SAA2. By binding assay on the microtiter plate, SAA had more affinity to fibronectin than those of heparan sulfate, collagen type I, or serum amyloid P component. These results indicate that fibronectin plays an important role in the development of amyloidosis by working as a linking protein between SAA and the cell surface of macrophages.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abankwa G. V., Ali-Khan Z. Alveolar hydatid cyst induced amyloid enhancing factor (AEF): physicochemical properties and abolition of AEF activity by serine protease inhibitors. Br J Exp Pathol. 1988 Feb;69(1):133–148. [PMC free article] [PubMed] [Google Scholar]
- Axelrad M. A., Kisilevsky R., Willmer J., Chen S. J., Skinner M. Further characterization of amyloid-enhancing factor. Lab Invest. 1982 Aug;47(2):139–146. [PubMed] [Google Scholar]
- Benditt E. P., Eriksen N., Hanson R. H. Amyloid protein SAA is an apoprotein of mouse plasma high density lipoprotein. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4092–4096. doi: 10.1073/pnas.76.8.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach S. M., Bhogal B., de Beer F. C., Black M. M., Pepys M. B. Primary localized cutaneous amyloidosis: dermal amyloid deposits do not bind antibodies to amyloid A protein, prealbumin or fibronectin. Br J Dermatol. 1982 Oct;107(4):453–459. doi: 10.1111/j.1365-2133.1982.tb00388.x. [DOI] [PubMed] [Google Scholar]
- Buckley P. J., Smith M. R., Braverman M. F., Dickson S. A. Human spleen contains phenotypic subsets of macrophages and dendritic cells that occupy discrete microanatomic locations. Am J Pathol. 1987 Sep;128(3):505–520. [PMC free article] [PubMed] [Google Scholar]
- Dahlbäck K., Löfberg H., Dahlbäck B. Immunohistochemical demonstration of vitronectin in association with elastin and amyloid deposits in human kidney. Histochemistry. 1987;87(6):511–515. doi: 10.1007/BF00492465. [DOI] [PubMed] [Google Scholar]
- Dixon A. J., Burns J., Dunnill M. S., McGee J. O. Distribution of fibronectin in normal and diseased human kidneys. J Clin Pathol. 1980 Nov;33(11):1021–1028. doi: 10.1136/jcp.33.11.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck R. F., Rogers S. L. Fibronectin is an acute phase reactant in mice. Clin Invest Med. 1985;8(2):148–151. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Gudewicz P. W., Molnar J., Lai M. Z., Beezhold D. W., Siefring G. E., Jr, Credo R. B., Lorand L. Fibronectin-mediated uptake of gelatin-coated latex particles by peritoneal macrophages. J Cell Biol. 1980 Nov;87(2 Pt 1):427–433. doi: 10.1083/jcb.87.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983 Mar 10;258(5):3332–3340. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Hoffman J. S., Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984 Feb 1;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J. H., Grennan D. Different macrophage populations distinguished by means of fluorescent polysaccharides. Recognition and properties of marginal-zone macrophages. Eur J Immunol. 1981 Mar;11(3):221–228. doi: 10.1002/eji.1830110311. [DOI] [PubMed] [Google Scholar]
- Isemura M., Sato N., Yamaguchi Y., Aikawa J., Munakata H., Hayashi N., Yosizawa Z., Nakamura T., Kubota A., Arakawa M. Isolation and characterization of fibronectin-binding proteoglycan carrying both heparan sulfate and dermatan sulfate chains from human placenta. J Biol Chem. 1987 Jun 25;262(18):8926–8933. [PubMed] [Google Scholar]
- Isemura M., Yamaguchi Y., Munakata H., Aikawa J., Kan M., Yamane I., Yosizawa Z. Isolation and characterization of human placenta fibronectin. J Biochem. 1984 Jul;96(1):163–169. doi: 10.1093/oxfordjournals.jbchem.a134808. [DOI] [PubMed] [Google Scholar]
- Kawahara E., Oda Y., Ooi A., Katsuda S., Nakanishi I., Umeda S. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors. A comparative study of immunoreactivity of GFAP, vimentin, S-100 protein, and neurofilament in 38 schwannomas and 18 neurofibromas. Am J Surg Pathol. 1988 Feb;12(2):115–120. doi: 10.1097/00000478-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Vaheri A., Roberts P. J., Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Invest. 1980 Jul;43(1):47–51. [PubMed] [Google Scholar]
- Linker A., Carney H. C. Presence and role of glycosaminoglycans in amyloidosis. Lab Invest. 1987 Sep;57(3):297–305. [PubMed] [Google Scholar]
- Martinez-Hernandez A. The hepatic extracellular matrix. II. Electron immunohistochemical studies in rats with CCl4-induced cirrhosis. Lab Invest. 1985 Aug;53(2):166–186. [PubMed] [Google Scholar]
- Minamoto T., Ooi A., Okada Y., Mai M., Nagai Y., Nakanishi I. Desmoplastic reaction of gastric carcinoma: a light- and electron-microscopic immunohistochemical analysis using collagen type-specific antibodies. Hum Pathol. 1988 Jul;19(7):815–821. doi: 10.1016/s0046-8177(88)80265-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dyck R. F., de Beer F. C., Skinner M., Cohen A. S. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol. 1979 Nov;38(2):284–293. [PMC free article] [PubMed] [Google Scholar]
- Rovin B., Molnar J., Chevalier D., Ng P. Interaction of plasma fibronectin (pFN) with membranous constituents of peritoneal exudate cells and pulmonary macrophages. J Leukoc Biol. 1984 Nov;36(5):601–620. doi: 10.1002/jlb.36.5.601. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Schmidt G., Robenek H., Harrach B., Glössl J., Nolte V., Hörmann H., Richter H., Kresse H. Interaction of small dermatan sulfate proteoglycan from fibroblasts with fibronectin. J Cell Biol. 1987 Jun;104(6):1683–1691. doi: 10.1083/jcb.104.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger M. J., McAdam K. P., Kaplan M. M., Sipe J. D., Vogel S. N., Rosenstreich D. L. Monokine-induced synthesis of serum amyloid A protein by hepatocytes. Nature. 1980 Jun 12;285(5765):498–500. doi: 10.1038/285498a0. [DOI] [PubMed] [Google Scholar]
- Shiroo M., Kawahara E., Nakanishi I., Migita S. Specific deposition of serum amyloid A protein 2 in the mouse. Scand J Immunol. 1987 Dec;26(6):709–716. doi: 10.1111/j.1365-3083.1987.tb02307.x. [DOI] [PubMed] [Google Scholar]
- Skogen B., Natvig J. B. Degradation of amyloid proteins by different serine proteases. Scand J Immunol. 1981 Oct;14(4):389–396. doi: 10.1111/j.1365-3083.1981.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R., Stephens C., Anastassiades T. Characterization of tissue and plasma glycosaminoglycans during experimental AA amyloidosis and acute inflammation. Qualitative and quantitative analysis. Lab Invest. 1987 Jun;56(6):665–675. [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R. Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis. A histochemical study. Lab Invest. 1985 Jul;53(1):37–44. [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEILUM G. PATHOGENESIS OF AMYLOIDOSIS. Acta Pathol Microbiol Scand. 1964;61:21–45. doi: 10.1111/apm.1964.61.1.21. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Vuento M., Salonen E., Pasanen M., Stenman U. H. Competitive enzyme immunoassay for human plasma fibronectin. J Immunol Methods. 1981;40(1):101–108. doi: 10.1016/0022-1759(81)90085-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Complete primary structures of two major murine serum amyloid A proteins deduced from cDNA sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2915–2919. doi: 10.1073/pnas.82.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Shiroo M., Migita S. Diverse gene expression for isotypes of murine serum amyloid A protein during acute phase reaction. Science. 1986 Apr 11;232(4747):227–229. doi: 10.1126/science.3456645. [DOI] [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Holford S., Feinstein A., Pepys M. B. Fibronectin and C4-binding protein are selectively bound by aggregated amyloid P component. J Exp Med. 1981 Oct 1;154(4):1134–1139. doi: 10.1084/jem.154.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]