Abstract
The extent to which human papillomavirus (HPV) type 16 is transcribed and the nature of the transcripts produced in genital precancers has not been clearly defined. The authors analyzed 28 cases of cervical (CIN) or vulvar (VIN) intraepithelial neoplasia by RNA-RNA in situ hybridization, using probes generated from HPV 16 open reading frames (ORFs) either upstream (E6-E7) or downstream (E2-E5-L2) to the E1 ORF, where HPV 16 genomic integration most commonly occurs. Hybridization signals corresponding to one or both probes were detected in a high proportion of cells throughout the lesional epithelium of low- and high-grade CIN, including basal layers. In serial sections analyzed with the two probes, hybridization signals were obtained from both, and in similar proportion, irrespective of CIN grade. The distribution and character of hybridization signals suggests that the morphologic progression of precancers is not associated with either cessation of HPV 16 early transcription or a change in the general character of the transcripts produced.
Full text
PDF
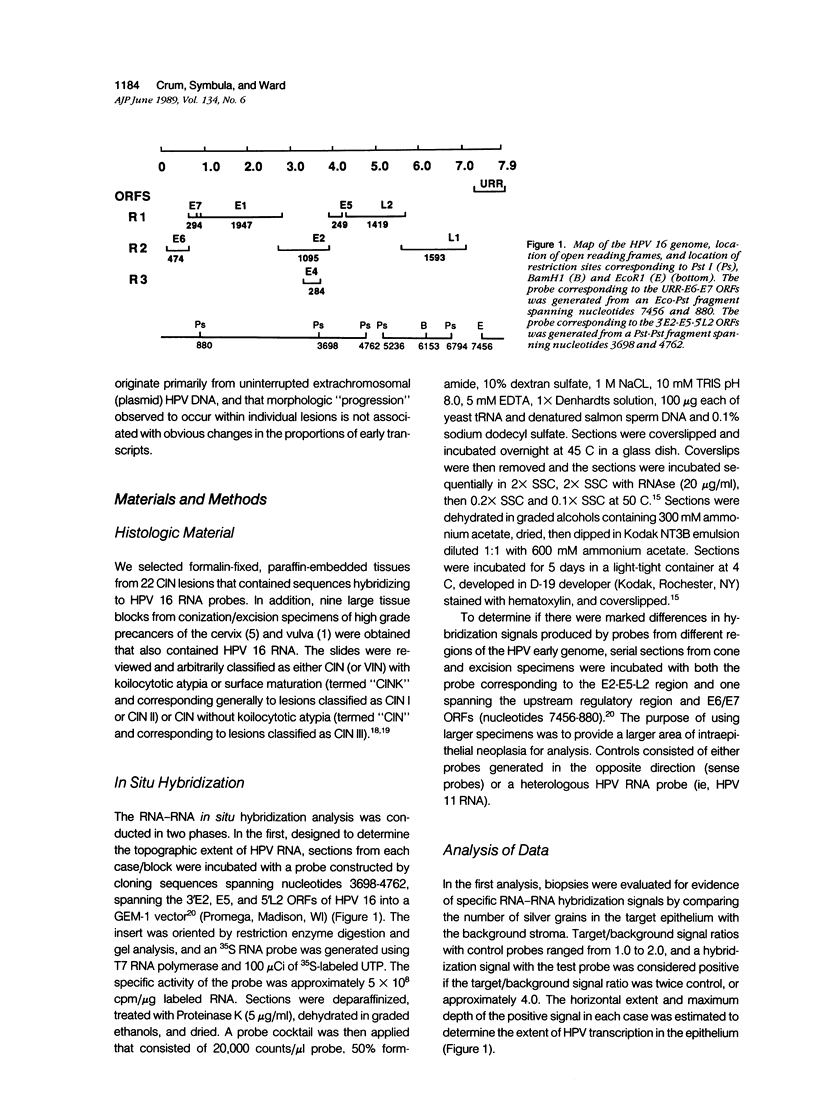

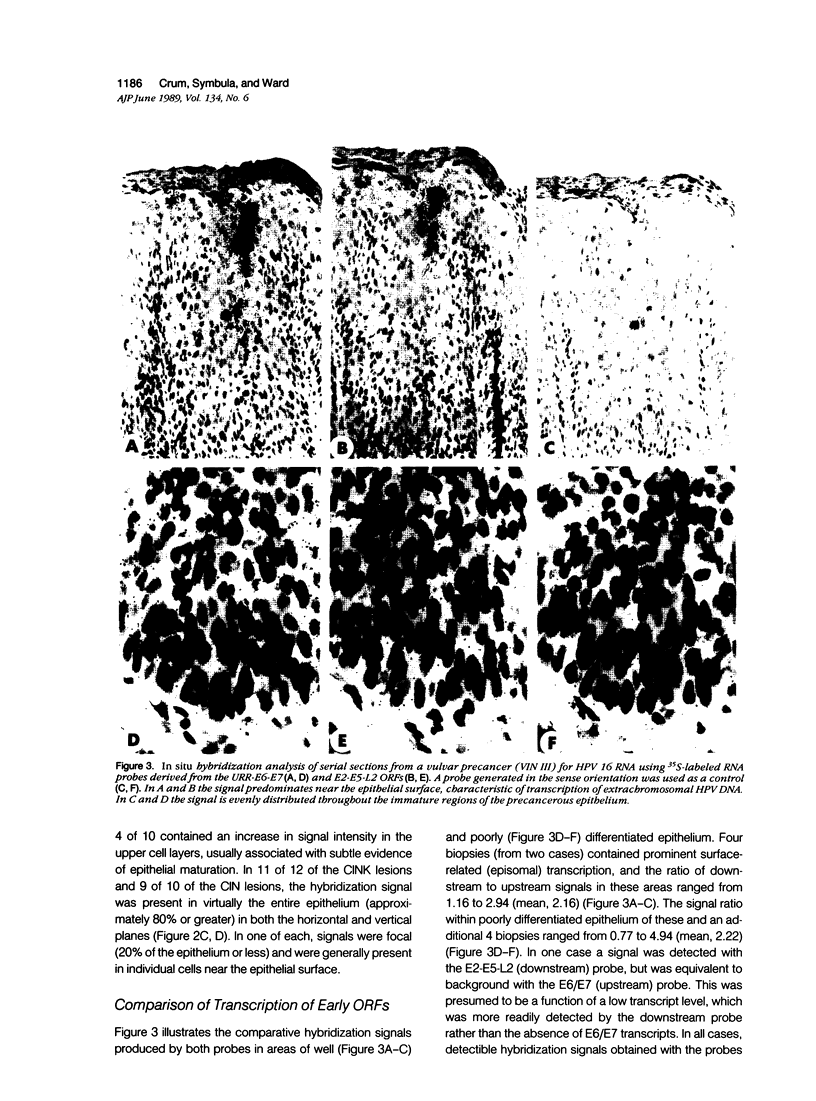


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Ikenberg H., Richart R. M., Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984 Apr 5;310(14):880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- Crum C. P., Mitao M., Levine R. U., Silverstein S. Cervical papillomaviruses segregate within morphologically distinct precancerous lesions. J Virol. 1985 Jun;54(3):675–681. doi: 10.1128/jvi.54.3.675-681.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum C. P., Nuovo G., Friedman D., Silverstein S. J. Accumulation of RNA homologous to human papillomavirus type 16 open reading frames in genital precancers. J Virol. 1988 Jan;62(1):84–90. doi: 10.1128/jvi.62.1.84-90.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J. Y., Defendi V. A viral-cellular junction fragment from a human papillomavirus type 16-positive tumor is competent in transformation of NIH 3T3 cells. J Virol. 1988 Nov;62(11):4420–4426. doi: 10.1128/jvi.62.11.4420-4426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn H., Villa L. L., Marziona F., Hilgarth M., Hillemans H. G., Sauer G. Physical state and biological activity of human papillomavirus genomes in precancerous lesions of the female genital tract. J Gen Virol. 1988 Jan;69(Pt 1):187–196. doi: 10.1099/0022-1317-69-1-187. [DOI] [PubMed] [Google Scholar]
- Lusky M., Botchan M. R. Characterization of the bovine papilloma virus plasmid maintenance sequences. Cell. 1984 Feb;36(2):391–401. doi: 10.1016/0092-8674(84)90232-0. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura T., Kanda T., Furuno A., Yoshikawa H., Kawana T., Yoshiike K. Cloning of monomeric human papillomavirus type 16 DNA integrated within cell DNA from a cervical carcinoma. J Virol. 1986 Jun;58(3):979–982. doi: 10.1128/jvi.58.3.979-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCance D. J., Kopan R., Fuchs E., Laimins L. A. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Maunoury S., Croissant O., Orth G. Integration of human papillomavirus type 16 DNA sequences: a possible early event in the progression of genital tumors. J Virol. 1987 Oct;61(10):3295–3298. doi: 10.1128/jvi.61.10.3295-3298.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Seedorf K., Krämmer G., Dürst M., Suhai S., Röwekamp W. G. Human papillomavirus type 16 DNA sequence. Virology. 1985 Aug;145(1):181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Kubota K., Kasai T., Sekiya S., Takamizawa H., Simizu B. Detection of human papillomavirus type 16 DNA and evidence for integration into the cell DNA in cervical dysplasia. J Gen Virol. 1986 Sep;67(Pt 9):2011–2015. doi: 10.1099/0022-1317-67-9-2011. [DOI] [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Kubota K., Kasai T., Sekiya S., Takamizawa H., Simizu B. Transcriptional differences of the human papillomavirus type 16 genome between precancerous lesions and invasive carcinomas. J Virol. 1988 Mar;62(3):1022–1027. doi: 10.1128/jvi.62.3.1022-1027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Broker T. R. In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum Pathol. 1986 Dec;17(12):1250–1258. doi: 10.1016/s0046-8177(86)80569-x. [DOI] [PubMed] [Google Scholar]