Abstract
Experimental nephrotic syndrome induced by several immunologic and biochemical methods is associated with the development of tubulointerstitial nephritis (TIN). To investigate the hypothesis that severe sustained proteinuria plays a role in the pathogenesis of TIN, the renal interstitium in a model of protein-overload proteinuria was studied. After uninephrectomy, rats received daily injections of 1.0 g of bovine serum albumin (BSA) or saline (controls) until killing at 1, 2, 4, or 7 weeks. Sections of frozen renal cortex were stained with a panel of monoclonal antibodies reactive with subsets of rat lymphohemopoietic cells, and positive tubulointerstitial cells (TIC) were quantitated by epifluorescence microscopy. BSA rats developed proteinuria, with mean rat urinary albumin excretion rates at 1, 2, 3, and 6 weeks of 35.6 +/- 21.8, 97.2 +/- 46.1, 63.6 +/- 40.8, and 58.6 +/- 24.4 mg/24 hours, respectively (controls, 0.17 +/- 0.16 mg/24 hours). BSA was detectable in the plasma of experimental animals at all periods, with mean values of 26.8 +/- 3.8, 27.8 +/- 2.7, 20.3 +/- 6.2, and 7.0 +/- 1.1 mg/ml (controls, 0.03 +/- 0.04 mg/ml) at 1, 2, 4, and 7 weeks, respectively, whereas plasma anti-BSA antibodies were never detected. A significant mononuclear cell infiltrate was present in the interstitium of experimental animals at all periods. At 1 week, an influx of macrophages was evident that was identified by surface markers OX42 (75+/1000 TIC) (P less than 0.01) and Ia (58+/1000 TIC) (P less than 0.01). Macrophages dominated the infiltrate at all periods. By 2 weeks, a significant population of lymphocytes was also present that was identified by the surface marker OX19 (54+/1000 TIC) (P less than 0.01). This early lymphocytic infiltrate was a mixed lesion of T helper and T cytotoxic cells. However, at 4 and 7 weeks, most lymphocytes expressed the OX8 cytotoxic T cell marker. The proximal tubules of proteinuric rats expressed vimentin intermediate filaments, a marker of tubular epithelial cell regeneration after injury. In BSA rats, C3 and neoantigens of the membrane attack complex of complement without IgG were present along the luminal border of many tubular epithelial cells. The interstitial infiltrate was confirmed by light microscopy. By 4 weeks, focal areas of chronic interstitial disease were evident consisting of tubular atrophy and interstitial fibrosis. In a second study, one group of BSA-treated rats was depleted of circulating T lymphocytes by daily parenteral injections of monoclonal antibody OX19.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



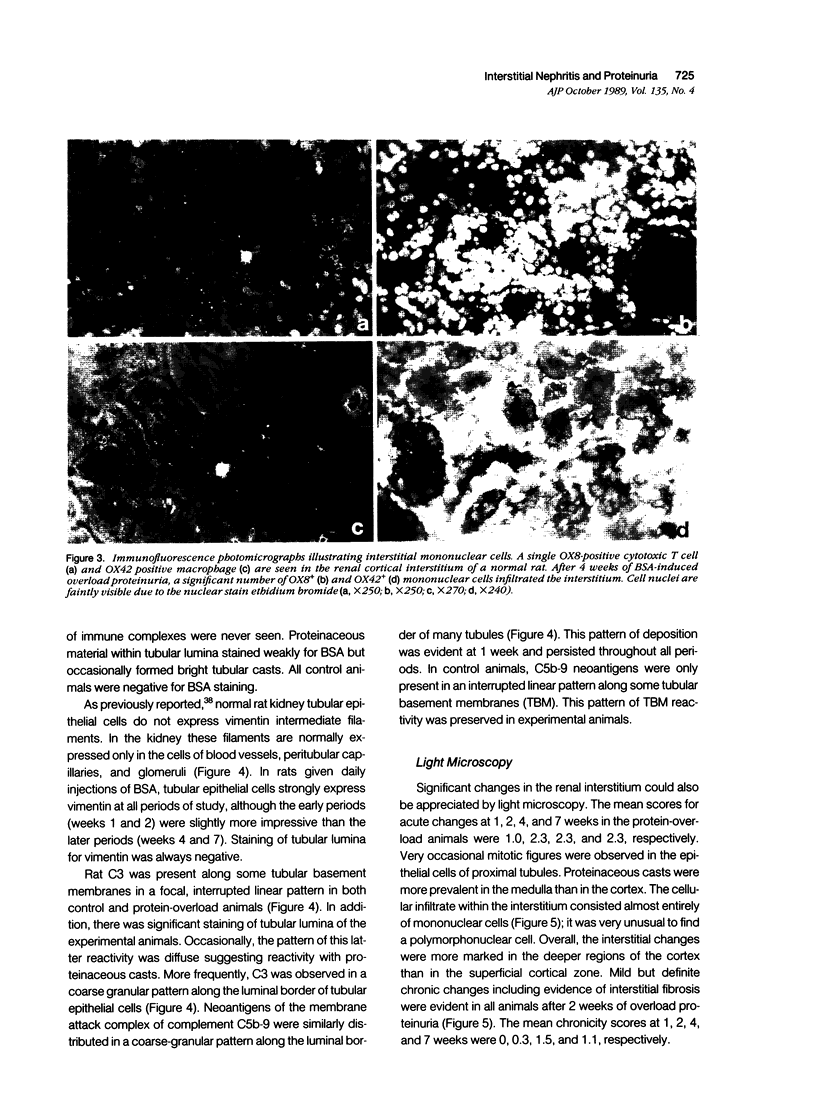
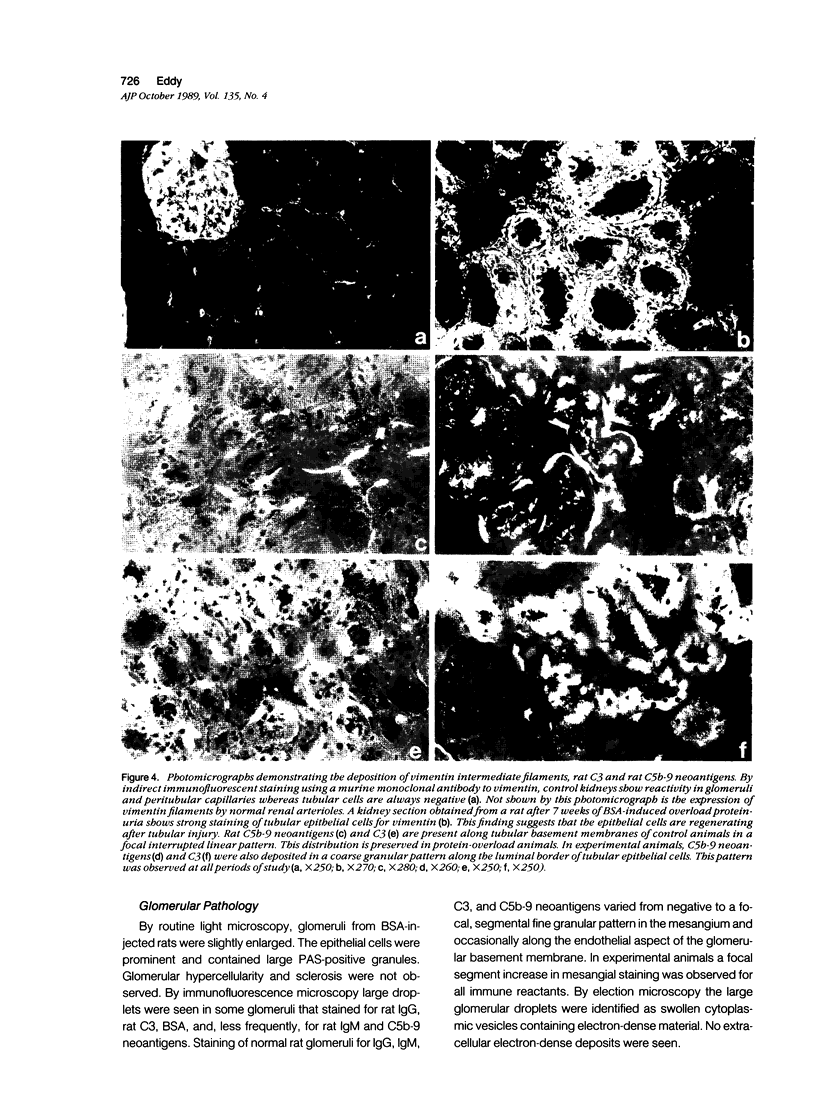


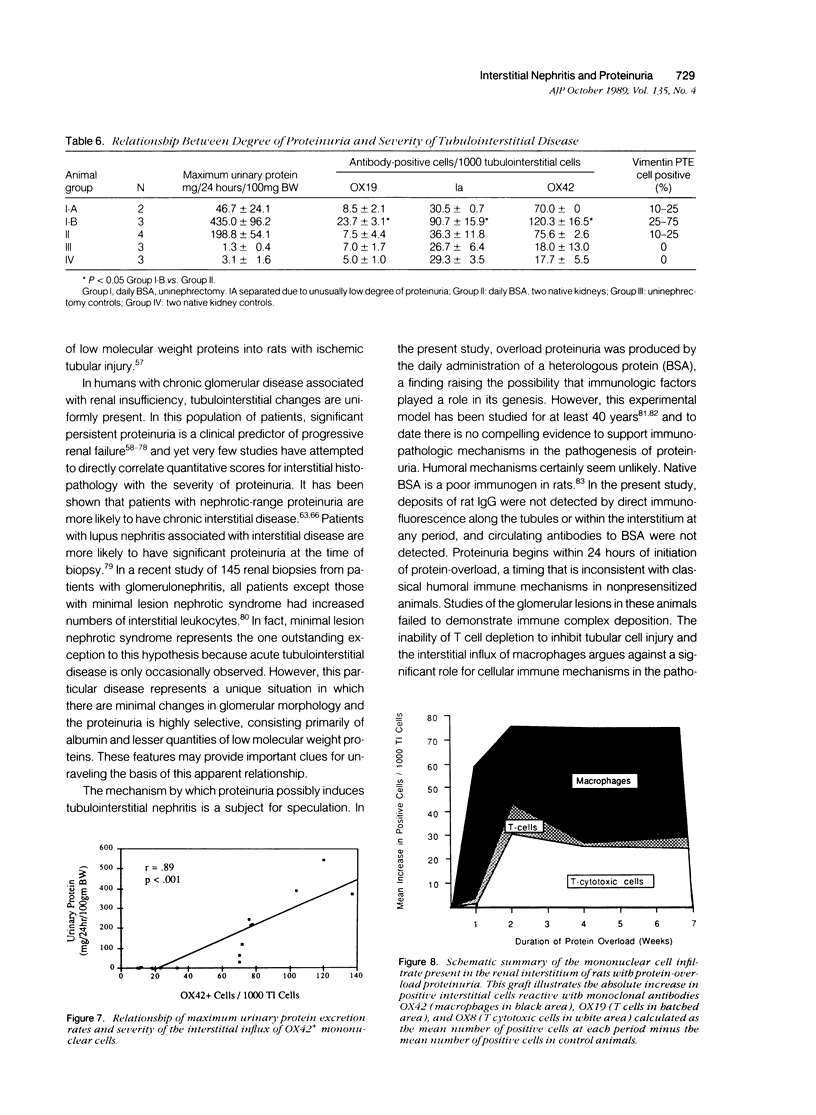




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON M. S., RECANT L. Fine structural alterations in the rat kidney following intraperitoneal bovine albumin. Am J Pathol. 1962 May;40:555–569. [PMC free article] [PubMed] [Google Scholar]
- Andrews P. M. A scanning and transmission electron microscopic comparison of puromycin aminonucleoside-induced nephrosis to hyperalbuminemia-induced proteinuria with emphasis on kidney podocyte pedicel loss. Lab Invest. 1977 Feb;36(2):183–197. [PubMed] [Google Scholar]
- Antoine B., Faye C. The clinical course associated with dense deposits in the kidney basement membranes. Kidney Int. 1972 Jun;1(6):420–427. doi: 10.1038/ki.1972.55. [DOI] [PubMed] [Google Scholar]
- Austin H. A., 3rd, Balow J. E. Henoch-Schönlein nephritis: prognostic features and the challenge of therapy. Am J Kidney Dis. 1983 Mar;2(5):512–520. doi: 10.1016/s0272-6386(83)80092-4. [DOI] [PubMed] [Google Scholar]
- Bagchus W. M., Hoedemaeker P. J., Rozing J., Bakker W. W. Glomerulonephritis induced by monoclonal anti-Thy 1.1 antibodies. A sequential histological and ultrastructural study in the rat. Lab Invest. 1986 Dec;55(6):680–687. [PubMed] [Google Scholar]
- Beaufils H., Alphonse J. C., Guedon J., Legrain M. Focal glomerulosclerosis: natural history and treatment. A report of 70 cases. Nephron. 1978;21(2):75–85. doi: 10.1159/000181374. [DOI] [PubMed] [Google Scholar]
- Bertani T., Cutillo F., Zoja C., Broggini M., Remuzzi G. Tubulo-interstitial lesions mediate renal damage in adriamycin glomerulopathy. Kidney Int. 1986 Oct;30(4):488–496. doi: 10.1038/ki.1986.212. [DOI] [PubMed] [Google Scholar]
- Bertani T., Rocchi G., Sacchi G., Mecca G., Remuzzi G. Adriamycin-induced glomerulosclerosis in the rat. Am J Kidney Dis. 1986 Jan;7(1):12–19. doi: 10.1016/s0272-6386(86)80051-8. [DOI] [PubMed] [Google Scholar]
- Biesecker G. Membrane attack complex of complement as a pathologic mediator. Lab Invest. 1983 Sep;49(3):237–249. [PubMed] [Google Scholar]
- Bohle A., Christ H., Grund K. E., Mackensen S. The role of the interstitium of the renal cortex in renal disease. Contrib Nephrol. 1979;16:109–114. doi: 10.1159/000402883. [DOI] [PubMed] [Google Scholar]
- Bohle A., von Gise H., Mackensen-Haen S., Stark-Jakob B. The obliteration of the postglomerular capillaries and its influence upon the function of both glomeruli and tubuli. Functional interpretation of morphologic findings. Klin Wochenschr. 1981 Sep 15;59(18):1043–1051. doi: 10.1007/BF01747747. [DOI] [PubMed] [Google Scholar]
- Bradley J. A., Mason D. W., Morris P. J. Evidence that rat renal allografts are rejected by cytotoxic T cells and not by nonspecific effectors. Transplantation. 1985 Feb;39(2):169–175. doi: 10.1097/00007890-198502000-00012. [DOI] [PubMed] [Google Scholar]
- Brideau R. J., Carter P. B., McMaster W. R., Mason D. W., Williams A. F. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980 Aug;10(8):609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- Cameron J. S., Turner D. R., Ogg C. S., Chantler C., Williams D. G. The long-term prognosis of patients with focal segmental glomerulosclerosis. Clin Nephrol. 1978 Dec;10(6):213–218. [PubMed] [Google Scholar]
- Camussi G., Rotunno M., Segoloni G., Brentjens J. R., Andres G. A. In vitro alternative pathway activation of complement by the brush border of proximal tubules of normal rat kidney. J Immunol. 1982 Apr;128(4):1659–1663. [PubMed] [Google Scholar]
- Clarkson A. R., Seymour A. E., Thompson A. J., Haynes W. D., Chan Y. L., Jackson B. IgA nephropathy: a syndrome of uniform morphology, diverse clinical features and uncertain prognosis. Clin Nephrol. 1977 Nov;8(5):459–471. [PubMed] [Google Scholar]
- Couser W. G., Baker P. J., Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985 Dec;28(6):879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- Croker B. P., Dawson D. V., Sanfilippo F. IgA nephropathy. Correlation of clinical and histologic features. Lab Invest. 1983 Jan;48(1):19–24. [PubMed] [Google Scholar]
- Dallman M. J., Mason D. W., Webb M. The roles of host and donor cells in the rejection of skin allografts by T cell-deprived rats injected with syngeneic T cells. Eur J Immunol. 1982 Jun;12(6):511–518. doi: 10.1002/eji.1830120612. [DOI] [PubMed] [Google Scholar]
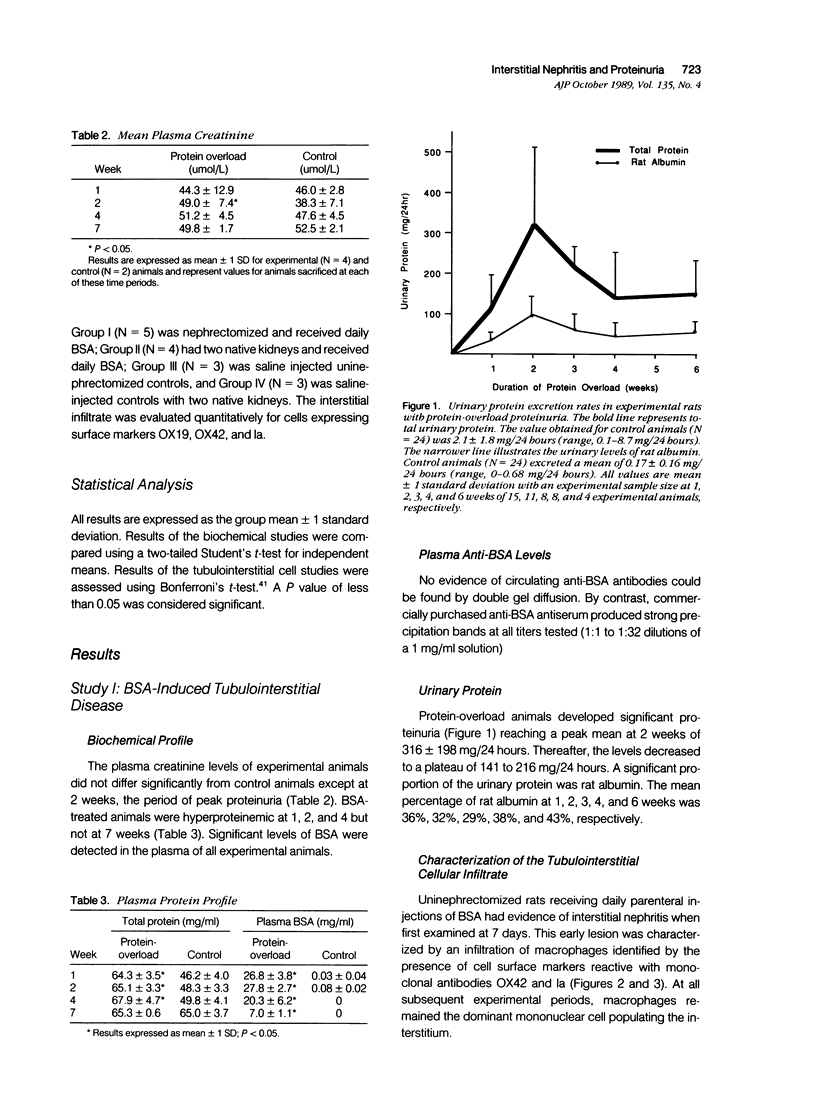
- Davies D. J., Brewer D. B., Hardwicke J. Urinary proteins and glomerular morphometry in protein overload proteinuria. Lab Invest. 1978 Mar;38(3):232–243. [PubMed] [Google Scholar]
- Davies D. J., Brewer D. B. Irreversible glomerular damage following heterologous serum albumin overload. J Pathol. 1977 Sep;123(1):45–52. doi: 10.1002/path.1711230106. [DOI] [PubMed] [Google Scholar]
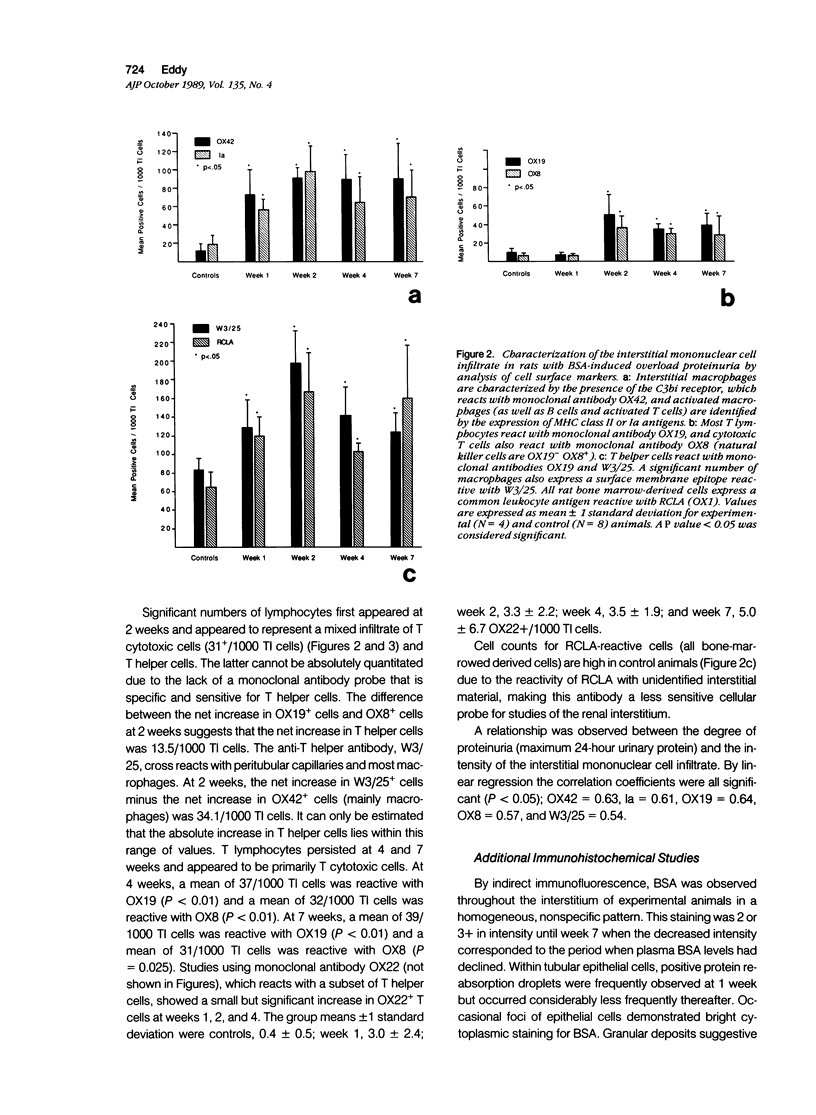
- Eddy A. A., Crary G. S., Michael A. F. Identification of lymphohemopoietic cells in the kidneys of normal rats. Am J Pathol. 1986 Aug;124(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Michael A. F. Acute tubulointerstitial nephritis associated with aminonucleoside nephrosis. Kidney Int. 1988 Jan;33(1):14–23. doi: 10.1038/ki.1988.3. [DOI] [PubMed] [Google Scholar]
- FISHER E. R., HELLSTROM H. R. Mechanism of proteinuria: Functional and ultrastructural correlation of effects of infusion of homologous and heterologous protein (bovine serum albumin) in the rat. Lab Invest. 1962 Aug;11:617–637. [PubMed] [Google Scholar]
- Giroux L., Smeesters C., Boury F., Faure M. P., Jean G. Adriamycin and adriamycin-DNA nephrotoxicity in rats. Lab Invest. 1984 Feb;50(2):190–196. [PubMed] [Google Scholar]
- Green J. R. Generation of cytotoxic T cells in the rat mixed lymphocyte reaction is blocked by monoclonal antibody MRC OX-8. Immunology. 1984 Jun;52(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Grégoire F. Kidney enzyme changes in experimental proteinuria. Lab Invest. 1971 Dec;25(6):626–634. [PubMed] [Google Scholar]
- Gröne H. J., Weber K., Gröne E., Helmchen U., Osborn M. Coexpression of keratin and vimentin in damaged and regenerating tubular epithelia of the kidney. Am J Pathol. 1987 Oct;129(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Habib R., Gubler M. C., Loirat C., Mäiz H. B., Levy M. Dense deposit disease: a variant of membranoproliferative glomerulonephritis. Kidney Int. 1975 Apr;7(4):204–215. doi: 10.1038/ki.1975.32. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Olsson T., Moran T., Klareskog L. In vivo treatment of rats with monoclonal anti-T-cell antibodies. Immunohistochemical and functional analysis in normal rats and in experimental allergic neuritis. Scand J Immunol. 1985 Aug;22(2):157–169. doi: 10.1111/j.1365-3083.1985.tb01868.x. [DOI] [PubMed] [Google Scholar]
- Hood S. A., Velosa J. A., Holley K. E., Donadio J. V., Jr IgA-IgG nephropathy: predictive indices of progressive disease. Clin Nephrol. 1981 Aug;16(2):55–62. [PubMed] [Google Scholar]
- Hooke D. H., Gee D. C., Atkins R. C. Leukocyte analysis using monoclonal antibodies in human glomerulonephritis. Kidney Int. 1987 Apr;31(4):964–972. doi: 10.1038/ki.1987.93. [DOI] [PubMed] [Google Scholar]
- Hyman L. R., Burkholder P. M. Focal sclerosing glomerulonephropathy with segmental hyalinosis. A clinicopathologic analysis. Lab Invest. 1973 May;28(5):533–544. [PubMed] [Google Scholar]
- KARL I. E., GARCIA P., WHITE W. L., RECANT L., KISSANE J. M. PROTEINURIA INDUCED BY ALBUMIN INJECTION; EFFECT ON KIDNEY AND LIVER ENZYMES IN THE RAT. Lab Invest. 1964 Dec;13:1600–1611. [PubMed] [Google Scholar]
- Katz A., Walker J. F., Landy P. J. IgA nephritis with nephrotic range proteinuria. Clin Nephrol. 1983 Aug;20(2):67–71. [PubMed] [Google Scholar]
- Kaysen G. A., Myers B. D., Couser W. G., Rabkin R., Felts J. M. Mechanisms and consequences of proteinuria. Lab Invest. 1986 May;54(5):479–498. [PubMed] [Google Scholar]
- Kobayashi Y., Tateno S., Hiki Y., Shigematsu H. IgA nephropathy: prognostic significance of proteinuria and histological alterations. Nephron. 1983;34(3):146–153. doi: 10.1159/000183000. [DOI] [PubMed] [Google Scholar]
- Lamb V., Tisher C. C., McCoy R. C., Robinson R. R. Membranoproliferative glomerulonephritis with dense intramembranous alterations. A clinicopathologic study. Lab Invest. 1977 Jun;36(6):607–617. [PubMed] [Google Scholar]
- Levy M., Broyer M., Arsan A., Levy-Bentolila D., Habib R. Anaphylactoid purpura nephritis in childhood: natural history and immunopathology. Adv Nephrol Necker Hosp. 1976;6:183–228. [PubMed] [Google Scholar]
- Like A. A., Biron C. A., Weringer E. J., Byman K., Sroczynski E., Guberski D. L. Prevention of diabetes in BioBreeding/Worcester rats with monoclonal antibodies that recognize T lymphocytes or natural killer cells. J Exp Med. 1986 Oct 1;164(4):1145–1159. doi: 10.1084/jem.164.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRISON A. B. Experimentally induced chronic renal insufficiency in the rat. Lab Invest. 1962 Apr;11:321–332. [PubMed] [Google Scholar]
- Maack T., Johnson V., Kau S. T., Figueiredo J., Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979 Sep;16(3):251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- Maack T., Mackensie D. D., Kinter W. B. Intracellular pathways of renal reabsorption of lysozyme. Am J Physiol. 1971 Dec;221(6):1609–1616. doi: 10.1152/ajplegacy.1971.221.6.1609. [DOI] [PubMed] [Google Scholar]
- Mackensen S., Grund K. E., Sindjić M., Bohle A. Influence of the renal cortical interstitium on the serum creatinine concentration and serum creatinine clearance in different chronic sclerosing interstitial nephritides. Nephron. 1979;24(1):30–34. doi: 10.1159/000181679. [DOI] [PubMed] [Google Scholar]
- Mampaso F. M., Wilson C. B. Characterization of inflammatory cells in autoimmune tubulointerstitial nephritis in rats. Kidney Int. 1983 Mar;23(3):448–457. doi: 10.1038/ki.1983.41. [DOI] [PubMed] [Google Scholar]
- Marks M. I., Drummond K. N. Nephropathy and persistent proteinuria after albumin administration in the rat. Lab Invest. 1970 Oct;23(4):416–420. [PubMed] [Google Scholar]
- Mason D. W., Arthur R. P., Dallman M. J., Green J. R., Spickett G. P., Thomas M. L. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- McMaster W. R., Williams A. F. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979 Jun;9(6):426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- Mori H., Yamashita H., Nakanishi C., Koizumi K., Makino S., Kishimoto Y., Hayashi Y. Proteinuria induced by transplantable rat pituitary tumor MtT SA5. Model for homologous protein-overload proteinuria. Lab Invest. 1986 Jun;54(6):636–644. [PubMed] [Google Scholar]
- Muckerheide A., Apple R. J., Pesce A. J., Michael J. G. Cationization of protein antigens. I. Alteration of immunogenic properties. J Immunol. 1987 Feb 1;138(3):833–837. [PubMed] [Google Scholar]
- Nagy J., Brasch H., Trinn C., Burger T. Clinical features and course of IgA glomerulonephritis. Acta Med Acad Sci Hung. 1982;39(3-4):201–210. [PubMed] [Google Scholar]
- Nagy J., Miltényi M., Dobos M., Burger T. Tubular proteinuria in IgA glomerulonephritis. Clin Nephrol. 1987 Feb;27(2):76–78. [PubMed] [Google Scholar]
- Nath K. A., Hostetter M. K., Hostetter T. H. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985 Aug;76(2):667–675. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel L. H., Zanetti M., Droz D., Barbanel C. Long-term prognosis of idiopathic membranous glomerulonephritis. Study of 116 untreated patients. Am J Med. 1979 Jan;66(1):82–90. doi: 10.1016/0002-9343(79)90486-8. [DOI] [PubMed] [Google Scholar]
- O'Dell J. R., Hays R. C., Guggenheim S. J., Steigerwald J. C. Tubulointerstitial renal disease in systemic lupus erythematosus. Arch Intern Med. 1985 Nov;145(11):1996–1999. [PubMed] [Google Scholar]
- O'Donnell M. P., Michels L., Kasiske B., Raij L., Keane W. F. Adriamycin-induced chronic proteinuria: a structural and functional study. J Lab Clin Med. 1985 Jul;106(1):62–67. [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. II. Prog Allergy. 1962;6:30–154. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- Okuda S., Oh Y., Tsuruda H., Onoyama K., Fujimi S., Fujishima M. Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int. 1986 Feb;29(2):502–510. doi: 10.1038/ki.1986.28. [DOI] [PubMed] [Google Scholar]
- Olbricht C. J., Cannon J. K., Garg L. C., Tisher C. C. Activities of cathepsins B and L in isolated nephron segments from proteinuric and nonproteinuric rats. Am J Physiol. 1986 Jun;250(6 Pt 2):F1055–F1062. doi: 10.1152/ajprenal.1986.250.6.F1055. [DOI] [PubMed] [Google Scholar]
- Olbricht C. J., Cannon J. K., Tisher C. C. Cathepsin B and L in nephron segments of rats with puromycin aminonucleoside nephrosis. Kidney Int. 1987 Sep;32(3):354–361. doi: 10.1038/ki.1987.217. [DOI] [PubMed] [Google Scholar]
- Park C. H., Maack T. Albumin absorption and catabolism by isolated perfused proximal convoluted tubules of the rabbit. J Clin Invest. 1984 Mar;73(3):767–777. doi: 10.1172/JCI111270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., D'Agati V., Appel G. B., Pirani C. L. Tubulointerstitial disease in lupus nephritis: relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44(4):309–319. doi: 10.1159/000184012. [DOI] [PubMed] [Google Scholar]
- Pesce A. J., Clyne D. H., Pollak V. E., Kant S. K., Foulkes E. C., Selenke W. M. Renal tubular interactions of proteins. Clin Biochem. 1980 Oct;13(5):209–215. doi: 10.1016/s0009-9120(80)80025-7. [DOI] [PubMed] [Google Scholar]
- Pierides A. M., Kerr D. N. Idiopathic membranous nephropathy. Nephron. 1978;20(6):301–303. doi: 10.1159/000181256. [DOI] [PubMed] [Google Scholar]
- Ponticelli C. Prognosis and treatment of membranous nephropathy. Kidney Int. 1986 Apr;29(4):927–940. doi: 10.1038/ki.1986.88. [DOI] [PubMed] [Google Scholar]
- Portman R. J., Kissane J. M., Robson A. M. Use of beta 2 microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986 Jul;30(1):91–98. doi: 10.1038/ki.1986.156. [DOI] [PubMed] [Google Scholar]
- Robinson A. P., White T. M., Mason D. W. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986 Feb;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Row P. G., Cameron J. S., Turner D. R., Evans D. J., White R. H., Ogg C. S., Chantler C., Brown C. B. Membranous nephropathy. Long-term follow-up and association with neoplasia. Q J Med. 1975 Apr;44(174):207–239. [PubMed] [Google Scholar]
- Sanders P. W., Herrera G. A., Chen A., Booker B. B., Galla J. H. Differential nephrotoxicity of low molecular weight proteins including Bence Jones proteins in the perfused rat nephron in vivo. J Clin Invest. 1988 Dec;82(6):2086–2096. doi: 10.1172/JCI113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schainuck L. I., Striker G. E., Cutler R. E., Benditt E. P. Structural-functional correlations in renal disease. II. The correlations. Hum Pathol. 1970 Dec;1(4):631–641. doi: 10.1016/s0046-8177(70)80061-2. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Harris K. P., Purkerson M. L., Klahr S. Immunological aspects of acute ureteral obstruction: immune cell infiltrate in the kidney. Kidney Int. 1988 Oct;34(4):487–493. doi: 10.1038/ki.1988.207. [DOI] [PubMed] [Google Scholar]
- Schwartz M. M., Bidani A. K., Lewis E. J. Glomerular epithelial cell structure and function in chronic proteinuria induced by homologous protein-load. Lab Invest. 1986 Dec;55(6):673–679. [PubMed] [Google Scholar]
- Schwartz M. M., Fennell J. S., Lewis E. J. Pathologic changes in the renal tubule in systemic lupus erythematosus. Hum Pathol. 1982 Jun;13(6):534–547. doi: 10.1016/s0046-8177(82)80268-2. [DOI] [PubMed] [Google Scholar]
- Spickett G. P., Brandon M. R., Mason D. W., Williams A. F., Woollett G. R. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983 Sep 1;158(3):795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur J Immunol. 1979 Feb;9(2):155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Green J. R. Molecular nature of the W3/25 and MRC OX-8 marker antigens for rat T lymphocytes: comparisons with mouse and human antigens. Eur J Immunol. 1983 Oct;13(10):855–858. doi: 10.1002/eji.1830131014. [DOI] [PubMed] [Google Scholar]
- Van Liew J. B., Brentjens J. R., Noble B. Relationship of kidney function to immunopathology in chronic serum sickness of rats. Kidney Int. 1983 Aug;24(2):160–169. doi: 10.1038/ki.1983.140. [DOI] [PubMed] [Google Scholar]
- Velosa J. A., Holley K. E., Torres V. E., Offord K. P. Significance of proteinuria on the outcome of renal function in patients with focal segmental glomerulosclerosis. Mayo Clin Proc. 1983 Sep;58(9):568–577. [PubMed] [Google Scholar]
- Weening J. J., Rennke H. G. Glomerular permeability and polyanion in adriamycin nephrosis in the rat. Kidney Int. 1983 Aug;24(2):152–159. doi: 10.1038/ki.1983.139. [DOI] [PubMed] [Google Scholar]
- Weiss M. A., Daquioag E., Margolin E. G., Pollak V. E. Nephrotic syndrome, progressive irreversible renal failure, and glomerular "collapse": a new clinicopathologic entity? Am J Kidney Dis. 1986 Jan;7(1):20–28. doi: 10.1016/s0272-6386(86)80052-x. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Galfrè G., Milstein C. Analysis of cell surfaces by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977 Nov;12(3):663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- Woda B. A., McFadden M. L., Welsh R. M., Bain K. M. Separation and isolation of rat natural killer (NK) cells from T cells with monoclonal antibodies. J Immunol. 1984 May;132(5):2183–2184. [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Matsubara K., Miyamoto H., Akano N., Maki S. Immunohistochemical studies of reflux nephropathy. The role of extracellular matrix, membrane attack complex, and immune cells in glomerular sclerosis. Am J Pathol. 1987 Nov;129(2):223–231. [PMC free article] [PubMed] [Google Scholar]
- Zager R. A., Teubner E. J., Adler S. Low molecular weight proteinuria exacerbates experimental ischemic renal injury. Lab Invest. 1987 Feb;56(2):180–188. [PubMed] [Google Scholar]