Abstract
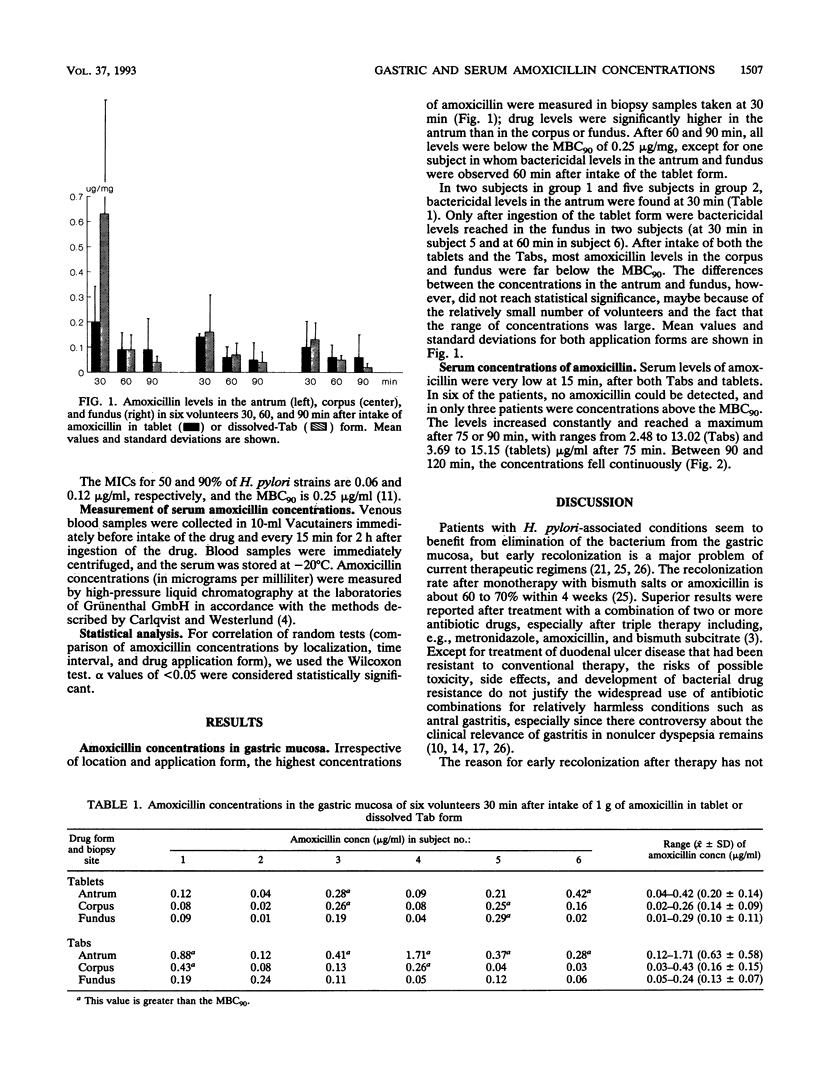
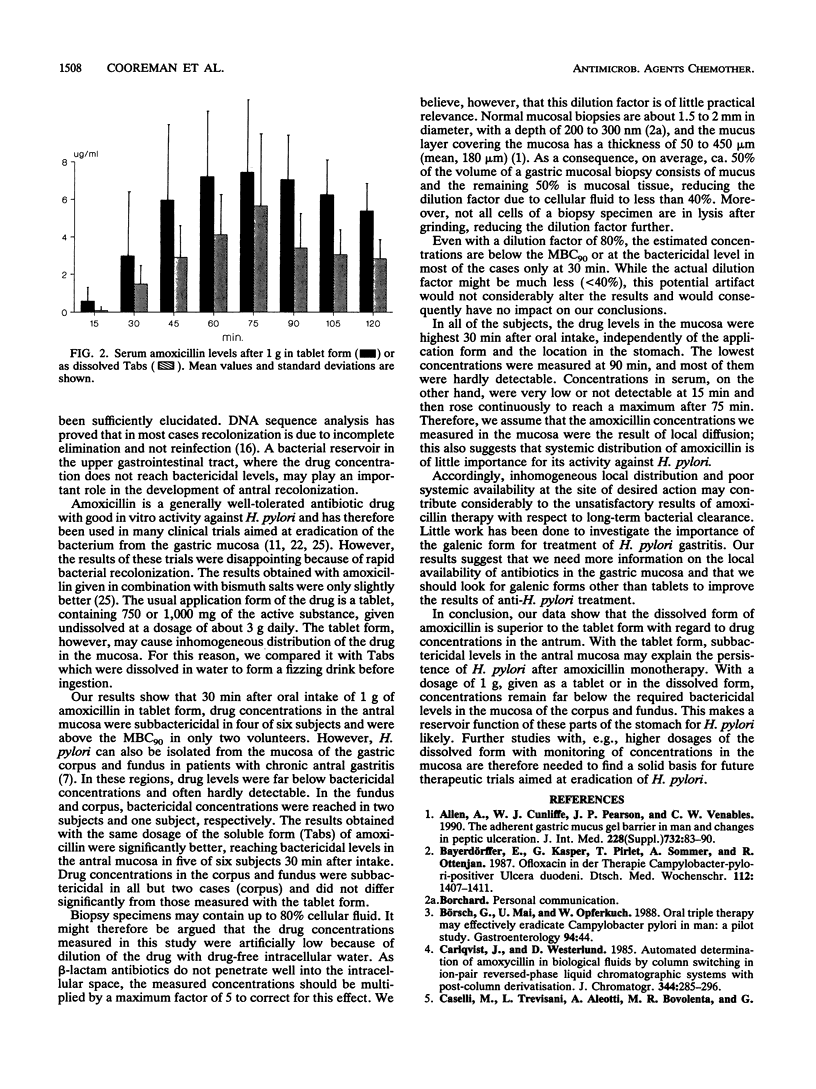
The high recolonization rate after monotherapy of Helicobacter pylori-positive gastritis may be due to insufficient local drug concentrations. To investigate the role of local diffusion, we measured levels of amoxicillin, a drug with good in vitro activity against H. pylori, in the mucosa and serum. One gram of amoxicillin was given to healthy volunteers as a tablet (n = 6) or as water-dissolved, fizzing "Tab" (n = 6). Gastroscopy with biopsies from the antrum, corpus, and fundus was performed at 30, 60, and 90 min. Concentrations in the mucosa were measured after homogenization with the agar diffusion method using Bacillus subtilis as the biological indicator. Serum samples, taken basally and every 15 min, were analyzed by high-pressure liquid chromatography. Drug levels in the fundus and corpus remained far below those in the antrum for both application forms. The highest concentrations were reached after 30 min, with bactericidal levels in the antrum in two of six subjects who took the tablet form and five of six subjects who used Tabs. At 60 and 90 min, almost all values were below the MBC for 90% of the strains tested. The concentrations in serum, however, rose continuously, to reach a maximum after 75 or 90 min. These results show that incomplete elimination may be due to subbactericidal concentrations of antibiotics with high in vitro efficiency at the desired site of action in vivo and that local diffusion in the mucosa is essential for therapeutic effectiveness against H. pylori.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Cunliffe W. J., Pearson J. P., Venables C. W. The adherent gastric mucus gel barrier in man and changes in peptic ulceration. J Intern Med Suppl. 1990;732:83–90. doi: 10.1111/j.1365-2796.1990.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Bayerdörffer E., Kasper G., Pirlet T., Sommer A., Ottenjann R. Ofloxacin in der Therapie Campylobacter-pylori-positiver Ulcera duodeni. Eine prospektive kontrollierte randomisierte Studie. Dtsch Med Wochenschr. 1987 Sep 11;112(37):1407–1411. doi: 10.1055/s-2008-1068260. [DOI] [PubMed] [Google Scholar]
- Carlqvist J., Westerlund D. Automated determination of amoxycillin in biological fluids by column switching in ion-pair reversed-phase liquid chromatographic systems with post-column derivatization. J Chromatogr. 1985 Nov 8;344:285–296. doi: 10.1016/s0378-4347(00)82029-0. [DOI] [PubMed] [Google Scholar]
- Caselli M., Trevisani L., Aleotti A., Bovolenta M. R., Stabellini G. Gastric metaplasia in duodenal bulb and Campylobacter-like organisms in development of duodenal ulcer. Dig Dis Sci. 1989 Sep;34(9):1374–1378. doi: 10.1007/BF01538072. [DOI] [PubMed] [Google Scholar]
- Coghlan J. G., Gilligan D., Humphries H., McKenna D., Dooley C., Sweeney E., Keane C., O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987 Nov 14;2(8568):1109–1111. doi: 10.1016/s0140-6736(87)91545-5. [DOI] [PubMed] [Google Scholar]
- Cohen H., Gramisu M., Fitzgibbons P., Appleman M., Skoglund M., Valenzuela J. E. Campylobacter pylori: associations with antral and fundic mucosal histology and diagnosis by serology in patients with upper gastrointestinal symptoms. Am J Gastroenterol. 1989 Apr;84(4):367–371. [PubMed] [Google Scholar]
- Dooley C. P., Cohen H., Fitzgibbons P. L., Bauer M., Appleman M. D., Perez-Perez G. I., Blaser M. J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989 Dec 7;321(23):1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- Drumm B., Sherman P., Cutz E., Karmali M. Association of Campylobacter pylori on the gastric mucosa with antral gastritis in children. N Engl J Med. 1987 Jun 18;316(25):1557–1561. doi: 10.1056/NEJM198706183162501. [DOI] [PubMed] [Google Scholar]
- Elta G. H., Murphy R., Behler E. M., Barnett J. L., Nostrant T. T., Kern S., Appelman H. Campylobacter pylori in patients with dyspeptic symptoms and endoscopic evidence of erosion(s). Am J Gastroenterol. 1989 Jun;84(6):643–646. [PubMed] [Google Scholar]
- Goodwin C. S., Blake P., Blincow E. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pyloridis. J Antimicrob Chemother. 1986 Mar;17(3):309–314. doi: 10.1093/jac/17.3.309. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Humphreys H., Bourke S., Dooley C., McKenna D., Power B., Keane C. T., Sweeney E. C., O'Moráin C. Effect of treatment on Campylobacter pylori in peptic disease: a randomised prospective trial. Gut. 1988 Mar;29(3):279–283. doi: 10.1136/gut.29.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. Y., Tay H. H., Wee A., Guan R., Math M. V., Yap I. Effect of colloidal bismuth subcitrate on symptoms and gastric histology in non-ulcer dyspepsia. A double blind placebo controlled study. Gut. 1990 Apr;31(4):476–480. doi: 10.1136/gut.31.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffeld R. J., Potters H. V., Stobberingh E., Flendrig J. A., van Spreeuwel J. P., Arends J. W. Campylobacter associated gastritis in patients with non-ulcer dyspepsia: a double blind placebo controlled trial with colloidal bismuth subcitrate. Gut. 1989 Sep;30(9):1206–1212. doi: 10.1136/gut.30.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffeld R. J., Stobberingh E., Flendrig J. A., van Spreeuwel J. P., Arends J. W. Diagnostic value of an immunoassay to detect anti Campylobacter pylori antibodies in non-ulcer dyspepsia. Lancet. 1989 May 27;1(8648):1182–1185. doi: 10.1016/s0140-6736(89)92761-x. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Goodwin C. S., Warren J. R., Murray R., Blincow E. D., Blackbourn S. J., Phillips M., Waters T. E., Sanderson C. R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988 Dec 24;2(8626-8627):1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Martin D. F., Montgomery E., Dobek A. S., Patrissi G. A., Peura D. A. Campylobacter pylori, NSAIDS, and smoking: risk factors for peptic ulcer disease. Am J Gastroenterol. 1989 Oct;84(10):1268–1272. [PubMed] [Google Scholar]
- McNulty C. A., Dent J., Wise R. Susceptibility of clinical isolates of Campylobacter pyloridis to 11 antimicrobial agents. Antimicrob Agents Chemother. 1985 Dec;28(6):837–838. doi: 10.1128/aac.28.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty C. A., Gearty J. C., Crump B., Davis M., Donovan I. A., Melikian V., Lister D. M., Wise R. Campylobacter pyloridis and associated gastritis: investigator blind, placebo controlled trial of bismuth salicylate and erythromycin ethylsuccinate. Br Med J (Clin Res Ed) 1986 Sep 13;293(6548):645–649. doi: 10.1136/bmj.293.6548.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Rokkas T., Pursey C., Uzoechina E., Dorrington L., Simmons N. A., Filipe M. I., Sladen G. E. Non-ulcer dyspepsia and short term De-Nol therapy: a placebo controlled trial with particular reference to the role of Campylobacter pylori. Gut. 1988 Oct;29(10):1386–1391. doi: 10.1136/gut.29.10.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolte M., Eidt S., Ritter M., Bethke B. Campylobacter pylori und Gastritis. Assoziation oder Induktion? Pathologe. 1989 Jan;10(1):21–26. [PubMed] [Google Scholar]


