Abstract
Regression of renal hyperplasia after withdrawal of the mitogenic stimulus induced by a single injection of lead nitrate was studied in male Wistar rats. Lead nitrate administration (10 mumol/100 g body weight) resulted in a ninefold increase in the incorporation of labeled thymidine into renal DNA and in an enhancement in the mitotic index; these changes were accompanied by an increase in the organ weight and DNA content that reached a maximum at 2 days. Regression of the renal hyperplasia was observed as early as 3 days after treatment and was completed within 2 weeks. Although lytic necrosis was not responsible for cell loss, the elimination of the excess renal cells took the form of apoptosis. This distinctive mode of cell death, which has been implicated in the involution of hyperplasia in other tissues and organs, was characterized by the occurrence of intracellular and extracellular membrane-bounded eosinophilic globules that often contained nuclear fragments. It affected mainly cells of the proximal tubules, and it was not detected once the kidney had regressed to its original mass. These results support the hypothesis that apoptosis is involved in the regulation of organ size.
Full text
PDF

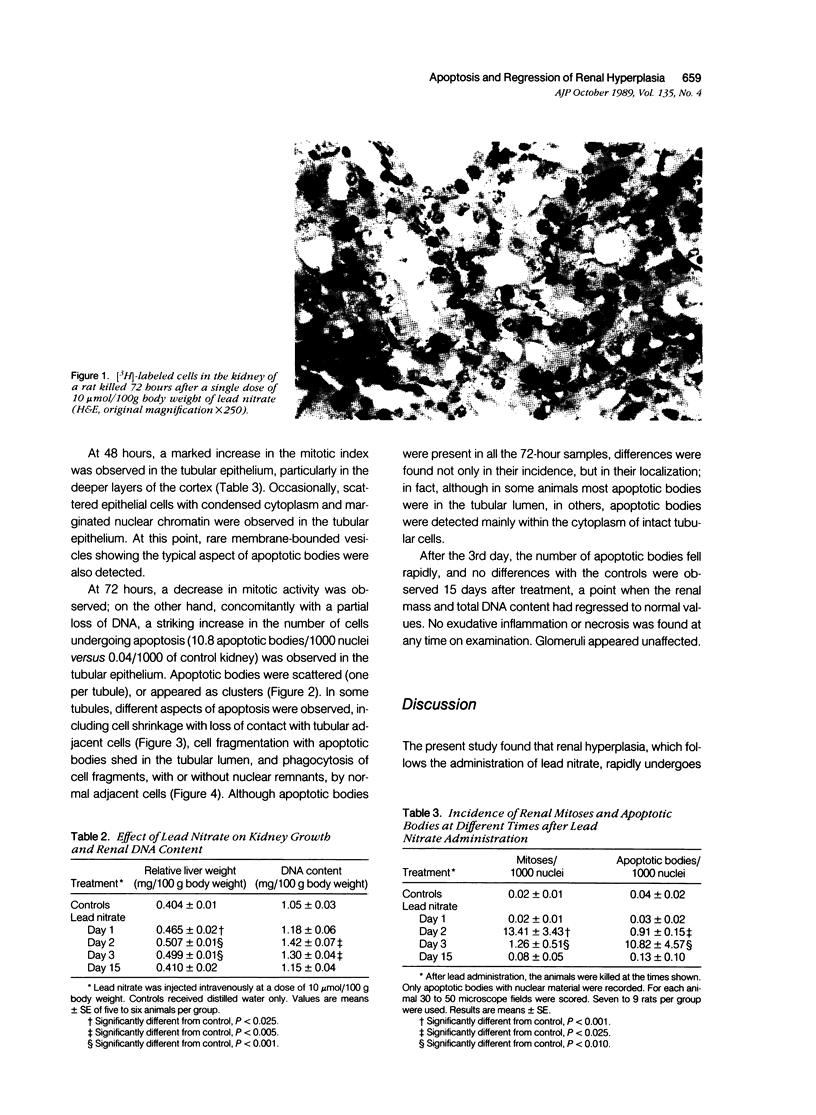



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azmi T. I., O'Shea J. D. Mechanism of deletion of endothelial cells during regression of the corpus luteum. Lab Invest. 1984 Aug;51(2):206–217. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellairs R. Cell death in chick embryos as studied by electron microscopy. J Anat. 1961 Jan;95(Pt 1):54–60.3. [PMC free article] [PubMed] [Google Scholar]
- Bhathal P. S., Gall J. A. Deletion of hyperplastic biliary epithelial cells by apoptosis following removal of the proliferative stimulus. Liver. 1985 Dec;5(6):311–325. doi: 10.1111/j.1600-0676.1985.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Bursch W., Lauer B., Timmermann-Trosiener I., Barthel G., Schuppler J., Schulte-Hermann R. Controlled death (apoptosis) of normal and putative preneoplastic cells in rat liver following withdrawal of tumor promoters. Carcinogenesis. 1984 Apr;5(4):453–458. doi: 10.1093/carcin/5.4.453. [DOI] [PubMed] [Google Scholar]
- Choie D. D., Richter G. W. Cell proliferation in rat kidney induced by lead acetate and effects of uninephrectomy on the proliferation. Am J Pathol. 1972 Feb;66(2):265–275. [PMC free article] [PubMed] [Google Scholar]
- Columbano A., Ledda-Columbano G. M., Rao P. M., Rajalakshmi S., Sarma D. S. Occurrence of cell death (apoptosis) in preneoplastic and neoplastic liver cells. A sequential study. Am J Pathol. 1984 Sep;116(3):441–446. [PMC free article] [PubMed] [Google Scholar]
- Decker R. S. Influence of thyroid hormones on neuronal death and differentiation in larval Rana pipiens. Dev Biol. 1976 Mar;49(1):101–118. doi: 10.1016/0012-1606(76)90261-x. [DOI] [PubMed] [Google Scholar]
- Gobe G. C., Axelsen R. A. Genesis of renal tubular atrophy in experimental hydronephrosis in the rat. Role of apoptosis. Lab Invest. 1987 Mar;56(3):273–281. [PubMed] [Google Scholar]
- Kerr J. F., Searle J. Deletion of cells by apoptosis during castration-induced involution of the rat prostate. Virchows Arch B Cell Pathol. 1973 Jun 25;13(2):87–102. doi: 10.1007/BF02889300. [DOI] [PubMed] [Google Scholar]
- Kerr J. F. Shrinkage necrosis: a distinct mode of cellular death. J Pathol. 1971 Sep;105(1):13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledda-Columbano G. M., Columbano A., Coni P., Curto M., Faa G., Pani P. Cell proliferation in rat kidney induced by 1,2-dibromoethane. Toxicol Lett. 1987 Jun;37(1):85–90. doi: 10.1016/0378-4274(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano G. M., Columbano A., Dessi S., Coni P., Chiodino C., Faa G., Pani P. Hexose monophosphate shunt and cholesterogenesis in lead-induced kidney hyperplasia. Chem Biol Interact. 1987;62(3):209–215. doi: 10.1016/0009-2797(87)90022-6. [DOI] [PubMed] [Google Scholar]
- Malamud D., Malt R. A. Stimulation of cell proliferation in mouse kidney by isoproterenol. Lab Invest. 1971 Feb;24(2):140–143. [PubMed] [Google Scholar]
- Potten C. S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977 Oct 6;269(5628):518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Taylor D. M., Threlfall G., Buck A. T. Stimulation of renal growth in the rat by folic acid. Nature. 1966 Oct 29;212(5061):472–474. doi: 10.1038/212472a0. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Walker N. I. Ultrastructure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol. 1987 Mar;126(3):439–451. [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death in the normal neonatal rat adrenal cortex. J Pathol. 1973 Dec;111(4):255–261. doi: 10.1002/path.1711110406. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]