Abstract
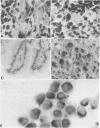
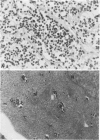
The two most recent hypotheses about the histogenesis of Ewing's Sarcoma (ES) are that it has a mesenchymal or neuroectodermal origin. Immunologic markers specific to these two tissue origins were tested on cryostat sections from three primary tumors carrying the chromosomal translocation t(11;22)(q24;q12). Cell lines established in vitro from two of these three primary tumors were also analyzed. Using antibodies directed against neural components (neurone-specific-enolase [NSE], HNK-1, and neurofilament triplet proteins [NFTP]), positive reactions were observed in cells from two primary tumors and their corresponding cell lines. Results of electron microscopic examination of the primary tumors were compatible with the diagnosis of ES. When using antibodies directed against mesenchymal cell surface antigens (common leucocytes, Leu M1, Leu M2, and Leu M3), the weak positive reactions observed in the three primary tumors were attributed to lymphoid infiltrates within tumor cells. Six additional ES cell lines carrying the translocation t(11;22) were also analyzed by immunocytochemical and flow cytometry methods using antibodies directed against mesenchymal and neural components. Positive reactions were observed in all seven cell lines tested using antibodies directed against NSE, HNK-1, and 200 KD subunit of the NFTP, whereas negative reactions were obtained with Leu M2 antibody. These results are consistent with a neuroectodermal origin of ES cells.
Full text
PDF
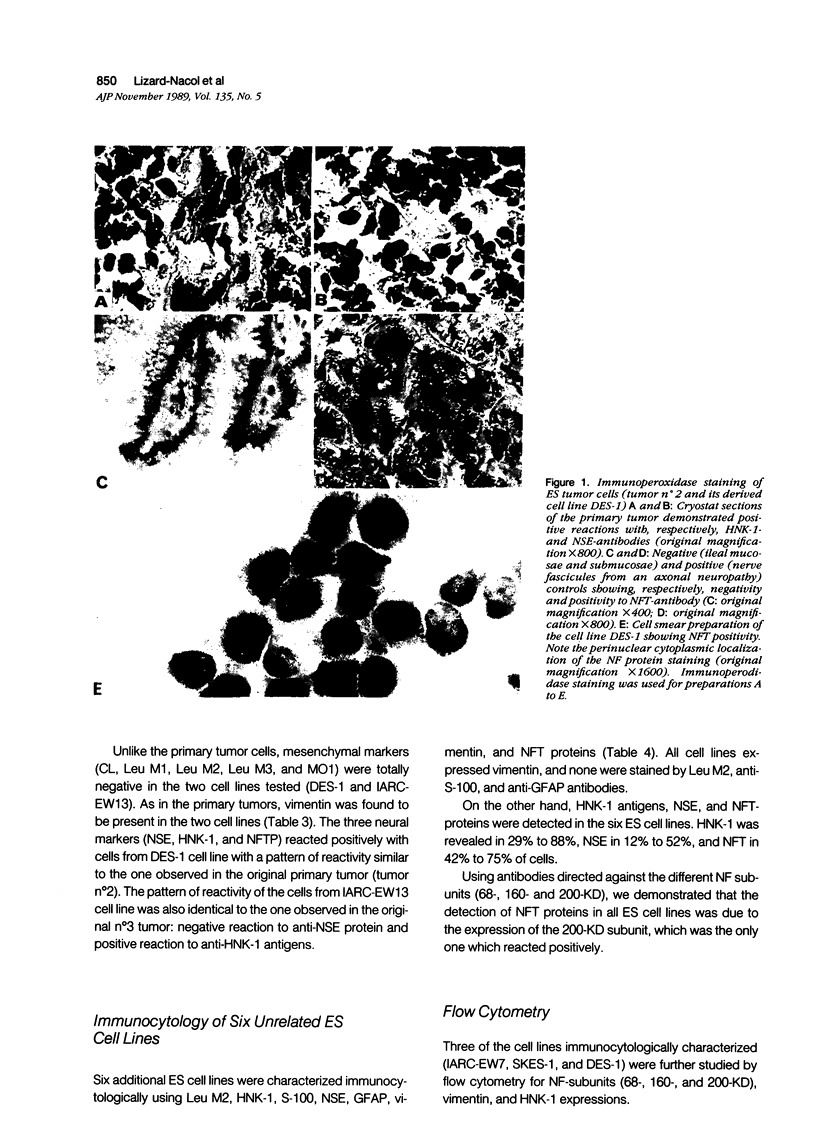
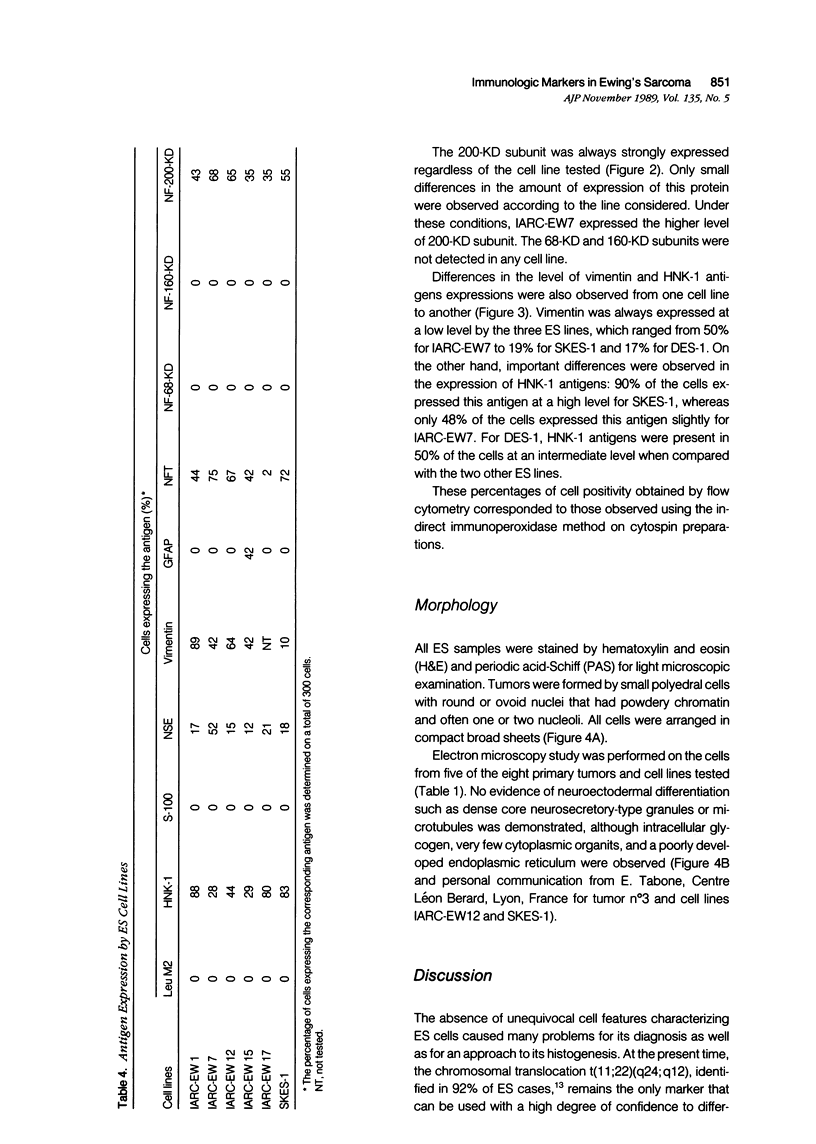
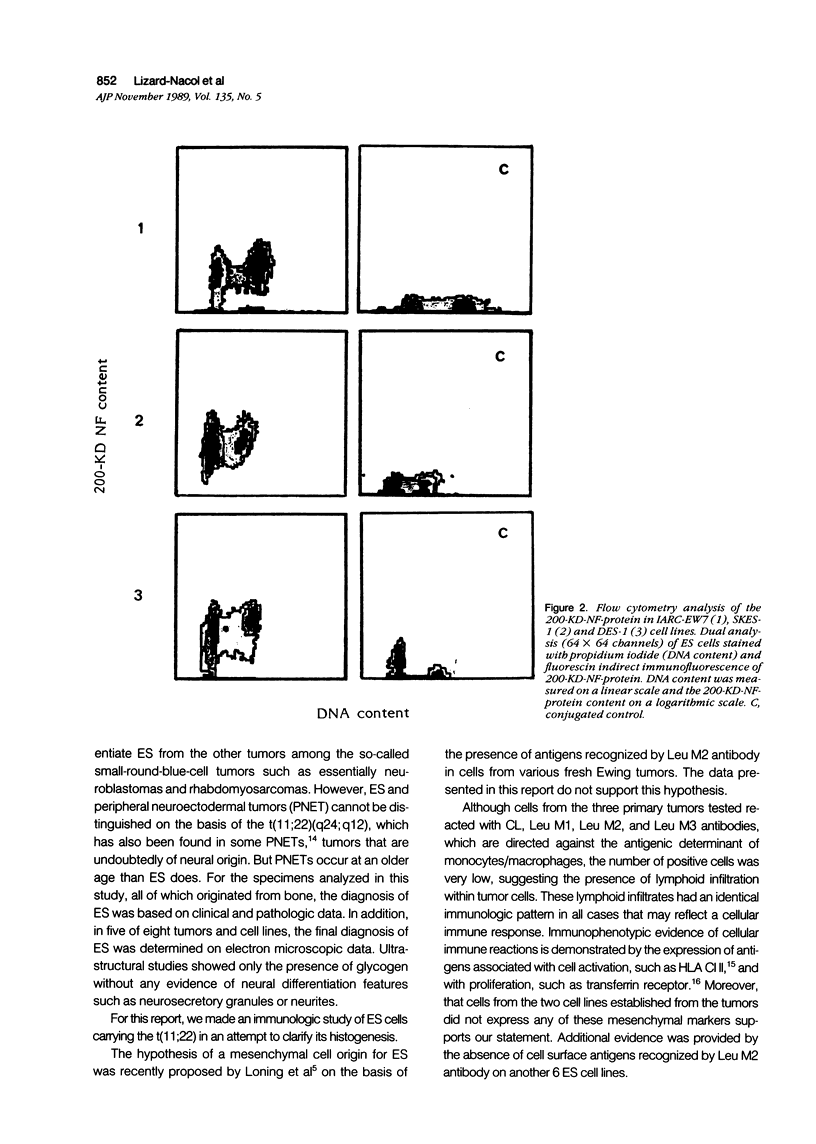


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks P. M. Diagnostic applications of an immunoperoxidase method in hematopathology. J Histochem Cytochem. 1979 Aug;27(8):1192–1194. doi: 10.1177/27.8.383825. [DOI] [PubMed] [Google Scholar]
- Bignami A., Raju T., Dahl D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol. 1982 Jun;91(2):286–295. doi: 10.1016/0012-1606(82)90035-5. [DOI] [PubMed] [Google Scholar]
- Bijman J. T., Wagener D. J., Wessels J. M., van den Broek P., Ramaekers F. C. Cell size, DNA, and cytokeratin analysis of human head and neck tumors by flow cytometry. Cytometry. 1986 Jan;7(1):76–81. doi: 10.1002/cyto.990070111. [DOI] [PubMed] [Google Scholar]
- Cavazzana A. O., Miser J. S., Jefferson J., Triche T. J. Experimental evidence for a neural origin of Ewing's sarcoma of bone. Am J Pathol. 1987 Jun;127(3):507–518. [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe R., Santamaria M., Yunis E. J., Tannery N. H., Agostini R. M., Jr, Medina J., Goodman M. The neuroectodermal tumor of bone. Am J Surg Pathol. 1984 Dec;8(12):885–898. doi: 10.1097/00000478-198412000-00001. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K., Koike M. Neuron-specific enolase and Leu-7 immunoreactive small round-cell neoplasm. The relationship to Ewing's sarcoma in bone and soft tissue. Am J Clin Pathol. 1986 Jul;86(1):79–83. doi: 10.1093/ajcp/86.1.79. [DOI] [PubMed] [Google Scholar]
- Lee V. M. Neurofilament protein abnormalities in PC12 cells: comparison with neurofilament proteins of normal cultured rat sympathetic neurons. J Neurosci. 1985 Nov;5(11):3039–3046. doi: 10.1523/JNEUROSCI.05-11-03039.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Braham K., Caillaud J. M., Carlu C., Tursz T. HNK-1 antibody detects an antigen expressed on neuroectodermal cells. J Exp Med. 1983 Nov 1;158(5):1775–1780. doi: 10.1084/jem.158.5.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M., Braham K., Philip I., Wiels J., Philip T., Goridis C., Lenoir G. M., Tursz T. Neuroectoderm-associated antigens on Ewing's sarcoma cell lines. Cancer Res. 1987 Jan 1;47(1):183–187. [PubMed] [Google Scholar]
- Löning T., Liebsch J., Delling G. Osteosarcomas and Ewing's sarcomas. Comparative immunocytochemical investigation of filamentous proteins and cell membrane determinants. Virchows Arch A Pathol Anat Histopathol. 1985;407(3):323–336. doi: 10.1007/BF00710657. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Histogenesis of Ewing's sarcoma. An evaluation of intermediate filaments and endothelial cell markers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(3):277–284. [PubMed] [Google Scholar]
- Moll R., Lee I., Gould V. E., Berndt R., Roessner A., Franke W. W. Immunocytochemical analysis of Ewing's tumors. Patterns of expression of intermediate filaments and desmosomal proteins indicate cell type heterogeneity and pluripotential differentiation. Am J Pathol. 1987 May;127(2):288–304. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Petzsch M., Vassallo J., Roessner A., Zwadlo G., Sorg C., Vollmer E., Grundmann E. Biological characterization of human bone tumors. VIII. Expression of HLA-DR antigens in bone tumors and tumor-like lesions. J Cancer Res Clin Oncol. 1986;112(2):144–150. doi: 10.1007/BF00404398. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Bennett G. S., Toyama Y., Kleinbart F., Holtzer H. Intermediate filament proteins in the developing chick spinal cord. Dev Biol. 1981 Aug;86(1):40–54. doi: 10.1016/0012-1606(81)90313-4. [DOI] [PubMed] [Google Scholar]
- Triche T. J., Askin F. B. Neuroblastoma and the differential diagnosis of small-, round-, blue-cell tumors. Hum Pathol. 1983 Jul;14(7):569–595. doi: 10.1016/s0046-8177(83)80202-0. [DOI] [PubMed] [Google Scholar]
- Trojanowski J. Q., Friedman H. S., Burger P. C., Bigner D. D. A rapidly dividing human medulloblastoma cell line (D283 MED) expresses all three neurofilament subunits. Am J Pathol. 1987 Feb;126(2):358–363. [PMC free article] [PubMed] [Google Scholar]
- Trojanowski J. Q. Neurofilament proteins and human nervous system tumors. J Histochem Cytochem. 1987 Sep;35(9):999–1003. doi: 10.1177/35.9.3611738. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C., Aurias A., Mugneret F., Lizard S., Sidaner I., Volk C., Thiery J. P., Olschwang S., Philip I., Berger M. P. Chromosomes in Ewing's sarcoma. I. An evaluation of 85 cases of remarkable consistency of t(11;22)(q24;q12). Cancer Genet Cytogenet. 1988 Jun;32(2):229–238. doi: 10.1016/0165-4608(88)90285-3. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Bonnin J. M., Rubinstein L. J., Marangos P. J. Immunohistochemical demonstration of neuron-specific enolase in neoplasms of the CNS and other tissues. Arch Pathol Lab Med. 1984 Jul;108(7):536–540. [PubMed] [Google Scholar]
- Wrba F., Ritzinger E., Reiner A., Holzner J. H. Transferrin receptor (TrfR) expression in breast carcinoma and its possible relationship to prognosis. An immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1986;410(1):69–73. doi: 10.1007/BF00710908. [DOI] [PubMed] [Google Scholar]