Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M., Damblon C., Plaitin B., Christiaens L., Frère J. M. Chromogenic depsipeptide substrates for beta-lactamases and penicillin-sensitive DD-peptidases. Biochem J. 1990 Sep 1;270(2):525–529. doi: 10.1042/bj2700525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N. E., Hobbs J. N., Jr, Richardson J. M., Riggin R. M. Biosynthesis of modified peptidoglycan precursors by vancomycin-resistant Enterococcus faecium. FEMS Microbiol Lett. 1992 Nov 1;77(1-3):109–115. doi: 10.1016/0378-1097(92)90140-j. [DOI] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Bugg T. D., Wright G. D., Walsh C. T., Courvalin P. Evidence for in vivo incorporation of D-lactate into peptidoglycan precursors of vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Apr;36(4):867–869. doi: 10.1128/aac.36.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Courvalin P. Sequence of the vanY gene required for production of a vancomycin-inducible D,D-carboxypeptidase in Enterococcus faecium BM4147. Gene. 1992 Oct 12;120(1):111–114. doi: 10.1016/0378-1119(92)90017-j. [DOI] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Courvalin P. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1992 Apr;174(8):2582–2591. doi: 10.1128/jb.174.8.2582-2591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
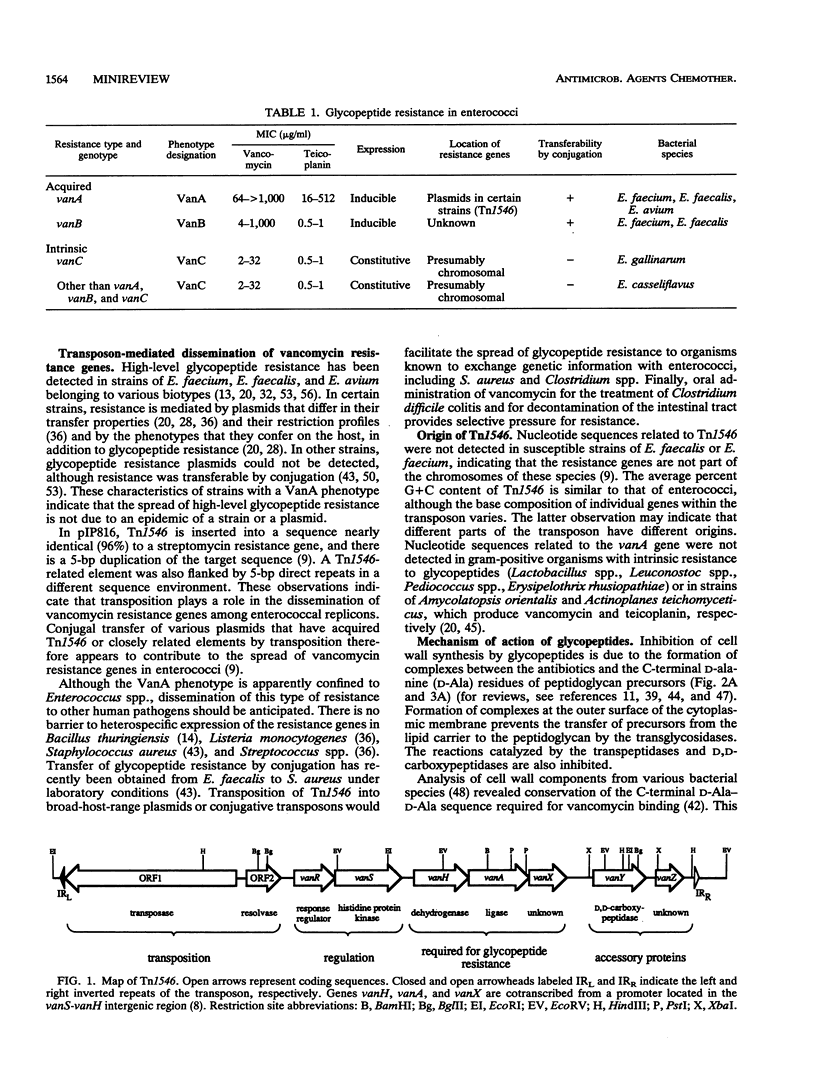
- Arthur M., Molinas C., Depardieu F., Courvalin P. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J Bacteriol. 1993 Jan;175(1):117–127. doi: 10.1128/jb.175.1.117-127.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur M., Molinas C., Dutka-Malen S., Courvalin P. Structural relationship between the vancomycin resistance protein VanH and 2-hydroxycarboxylic acid dehydrogenases. Gene. 1991 Jul 15;103(1):133–134. doi: 10.1016/0378-1119(91)90405-z. [DOI] [PubMed] [Google Scholar]
- Barna J. C., Williams D. H. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu Rev Microbiol. 1984;38:339–357. doi: 10.1146/annurev.mi.38.100184.002011. [DOI] [PubMed] [Google Scholar]
- Billot-Klein D., Gutmann L., Collatz E., van Heijenoort J. Analysis of peptidoglycan precursors in vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Jul;36(7):1487–1490. doi: 10.1128/aac.36.7.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991 Sep;29(9):1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noël A., Dutka-Malen S., Molinas C., Leclercq R., Courvalin P. Cloning and heterospecific expression of the resistance determinant vanA encoding high-level resistance to glycopeptides in Enterococcus faecium BM4147. Antimicrob Agents Chemother. 1990 May;34(5):924–927. doi: 10.1128/aac.34.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg T. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Identification of vancomycin resistance protein VanA as a D-alanine:D-alanine ligase of altered substrate specificity. Biochemistry. 1991 Feb 26;30(8):2017–2021. doi: 10.1021/bi00222a002. [DOI] [PubMed] [Google Scholar]
- Bugg T. D., Wright G. D., Dutka-Malen S., Arthur M., Courvalin P., Walsh C. T. Molecular basis for vancomycin resistance in Enterococcus faecium BM4147: biosynthesis of a depsipeptide peptidoglycan precursor by vancomycin resistance proteins VanH and VanA. Biochemistry. 1991 Oct 29;30(43):10408–10415. doi: 10.1021/bi00107a007. [DOI] [PubMed] [Google Scholar]
- Cercenado E., Eliopoulos G. M., Wennersten C. B., Moellering R. C., Jr Absence of synergistic activity between ampicillin and vancomycin against highly vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Oct;36(10):2201–2203. doi: 10.1128/aac.36.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. Resistance of enterococci to glycopeptides. Antimicrob Agents Chemother. 1990 Dec;34(12):2291–2296. doi: 10.1128/aac.34.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. Sequence of the vanC gene of Enterococcus gallinarum BM4174 encoding a D-alanine:D-alanine ligase-related protein necessary for vancomycin resistance. Gene. 1992 Mar 1;112(1):53–58. doi: 10.1016/0378-1119(92)90302-6. [DOI] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. The VANA glycopeptide resistance protein is related to D-alanyl-D-alanine ligase cell wall biosynthesis enzymes. Mol Gen Genet. 1990 Dec;224(3):364–372. doi: 10.1007/BF00262430. [DOI] [PubMed] [Google Scholar]
- Evers S., Sahm D. F., Courvalin P. The vanB gene of vancomycin-resistant Enterococcus faecalis V583 is structurally related to genes encoding D-Ala:D-Ala ligases and glycopeptide-resistance proteins VanA and VanC. Gene. 1993 Feb 14;124(1):143–144. doi: 10.1016/0378-1119(93)90779-3. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Billot-Klein D., al-Obeid S., Klare I., Francoual S., Collatz E., van Heijenoort J. Inducible carboxypeptidase activity in vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992 Jan;36(1):77–80. doi: 10.1128/aac.36.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Discotto L., Thanassi J., Pucci M. J. Insertional inactivation of a gene which controls expression of vancomycin resistance on plasmid pHKK100. FEMS Microbiol Lett. 1992 Apr 1;71(1):11–14. doi: 10.1016/0378-1097(92)90533-t. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Kolokathis A. Induction of vancomycin resistance in Enterococcus faecium by inhibition of transglycosylation. FEMS Microbiol Lett. 1990 Jul;58(2):167–170. doi: 10.1111/j.1574-6968.1990.tb13972.x. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Perlman D. C., Altarac D., McAuliffe V. Concomitant high-level vancomycin and penicillin resistance in clinical isolates of enterococci. Clin Infect Dis. 1992 Mar;14(3):655–661. doi: 10.1093/clinids/14.3.655. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Pucci M. J., Kolokathis A. Vancomycin resistance is encoded on a pheromone response plasmid in Enterococcus faecium 228. Antimicrob Agents Chemother. 1990 Feb;34(2):358–360. doi: 10.1128/aac.34.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Pucci M. J., Volk K. J., Liu J., Lee M. S. The cytoplasmic peptidoglycan precursor of vancomycin-resistant Enterococcus faecalis terminates in lactate. J Bacteriol. 1992 Sep;174(18):5982–5984. doi: 10.1128/jb.174.18.5982-5984.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden M. K., Trenholme G. M., Schultz J. E., Sahm D. F. In vivo development of teicoplanin resistance in a VanB Enterococcus faecium isolate. J Infect Dis. 1993 May;167(5):1224–1227. doi: 10.1093/infdis/167.5.1224. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Uttley A. H., Woodford N., George R. C. Resistance to vancomycin and teicoplanin: an emerging clinical problem. Clin Microbiol Rev. 1990 Jul;3(3):280–291. doi: 10.1128/cmr.3.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare I., Heier H., Claus H., Witte W. Environmental strains of Enterococcus faecium with inducible high-level resistance to glycopeptides. FEMS Microbiol Lett. 1993 Jan 1;106(1):23–29. doi: 10.1111/j.1574-6968.1993.tb05930.x. [DOI] [PubMed] [Google Scholar]
- Knox J. R., Pratt R. F. Different modes of vancomycin and D-alanyl-D-alanine peptidase binding to cell wall peptide and a possible role for the vancomycin resistance protein. Antimicrob Agents Chemother. 1990 Jul;34(7):1342–1347. doi: 10.1128/aac.34.7.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Bingen E., Su Q. H., Lambert-Zechovski N., Courvalin P., Duval J. Effects of combinations of beta-lactams, daptomycin, gentamicin, and glycopeptides against glycopeptide-resistant enterococci. Antimicrob Agents Chemother. 1991 Jan;35(1):92–98. doi: 10.1128/aac.35.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Weber M., Duval J., Courvalin P. Transferable vancomycin and teicoplanin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1989 Jan;33(1):10–15. doi: 10.1128/aac.33.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Dutka-Malen S., Duval J., Courvalin P. Vancomycin resistance gene vanC is specific to Enterococcus gallinarum. Antimicrob Agents Chemother. 1992 Sep;36(9):2005–2008. doi: 10.1128/aac.36.9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer J., Reynolds P. E. Modified peptidoglycan precursors produced by glycopeptide-resistant enterococci. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):195–200. doi: 10.1016/0378-1097(92)90608-q. [DOI] [PubMed] [Google Scholar]
- Nagarajan R. Antibacterial activities and modes of action of vancomycin and related glycopeptides. Antimicrob Agents Chemother. 1991 Apr;35(4):605–609. doi: 10.1128/aac.35.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Cole C. T., Preston D. A., Schabel A. A., Nagarajan R. Activity of glycopeptides against vancomycin-resistant gram-positive bacteria. Antimicrob Agents Chemother. 1989 Sep;33(9):1477–1481. doi: 10.1128/aac.33.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Wu C. Y., Hobbs J. N., Jr, Preston D. A., Allen N. E. Characterization of vancomycin resistance in Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother. 1989 Jul;33(7):1121–1124. doi: 10.1128/aac.33.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble W. C., Virani Z., Cree R. G. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett. 1992 Jun 1;72(2):195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- Quintiliani R., Jr, Evers S., Courvalin P. The vanB gene confers various levels of self-transferable resistance to vancomycin in enterococci. J Infect Dis. 1993 May;167(5):1220–1223. doi: 10.1093/infdis/167.5.1220. [DOI] [PubMed] [Google Scholar]
- Rasmussen J. R., Strominger J. L. Utilization of a depsipeptide substrate for trapping acyl-enzyme intermediates of penicillin-sensitive D-alanine carboxypeptidases. Proc Natl Acad Sci U S A. 1978 Jan;75(1):84–88. doi: 10.1073/pnas.75.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989 Nov;8(11):943–950. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Etter L., Gutmann L. Synergistic killing of vancomycin-resistant enterococci of classes A, B, and C by combinations of vancomycin, penicillin, and gentamicin. Antimicrob Agents Chemother. 1991 Apr;35(4):776–779. doi: 10.1128/aac.35.4.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttley A. H., George R. C., Naidoo J., Woodford N., Johnson A. P., Collins C. H., Morrison D., Gilfillan A. J., Fitch L. E., Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989 Aug;103(1):173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. T. Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem. 1989 Feb 15;264(5):2393–2396. [PubMed] [Google Scholar]
- Williamson R., Al-Obeid S., Shlaes J. H., Goldstein F. W., Shlaes D. M. Inducible resistance to vancomycin in Enterococcus faecium D366. J Infect Dis. 1989 Jun;159(6):1095–1104. doi: 10.1093/infdis/159.6.1095. [DOI] [PubMed] [Google Scholar]
- Woodford N., Morrison D., Johnson A. P., Briant V., George R. C., Cookson B. Application of DNA probes for rRNA and vanA genes to investigation of a nosocomial cluster of vancomycin-resistant enterococci. J Clin Microbiol. 1993 Mar;31(3):653–658. doi: 10.1128/jcm.31.3.653-658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. D., Holman T. R., Walsh C. T. Purification and characterization of VanR and the cytosolic domain of VanS: a two-component regulatory system required for vancomycin resistance in Enterococcus faecium BM4147. Biochemistry. 1993 May 18;32(19):5057–5063. doi: 10.1021/bi00070a013. [DOI] [PubMed] [Google Scholar]
- Wright G. D., Molinas C., Arthur M., Courvalin P., Walsh C. T. Characterization of vanY, a DD-carboxypeptidase from vancomycin-resistant Enterococcus faecium BM4147. Antimicrob Agents Chemother. 1992 Jul;36(7):1514–1518. doi: 10.1128/aac.36.7.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Obeid S., Billot-Klein D., van Heijenoort J., Collatz E., Gutmann L. Replacement of the essential penicillin-binding protein 5 by high-molecular mass PBPs may explain vancomycin-beta-lactam synergy in low-level vancomycin-resistant Enterococcus faecium D366. FEMS Microbiol Lett. 1992 Feb 1;70(1):79–84. doi: 10.1016/0378-1097(92)90566-7. [DOI] [PubMed] [Google Scholar]
- al-Obeid S., Collatz E., Gutmann L. Mechanism of resistance to vancomycin in Enterococcus faecium D366 and Enterococcus faecalis A256. Antimicrob Agents Chemother. 1990 Feb;34(2):252–256. doi: 10.1128/aac.34.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Obeid S., Gutmann L., Shlaes D. M., Williamson R., Collatz E. Comparison of vancomycin-inducible proteins from four strains of Enterococci. FEMS Microbiol Lett. 1990 Jun 15;58(1):101–105. doi: 10.1016/0378-1097(90)90110-c. [DOI] [PubMed] [Google Scholar]


