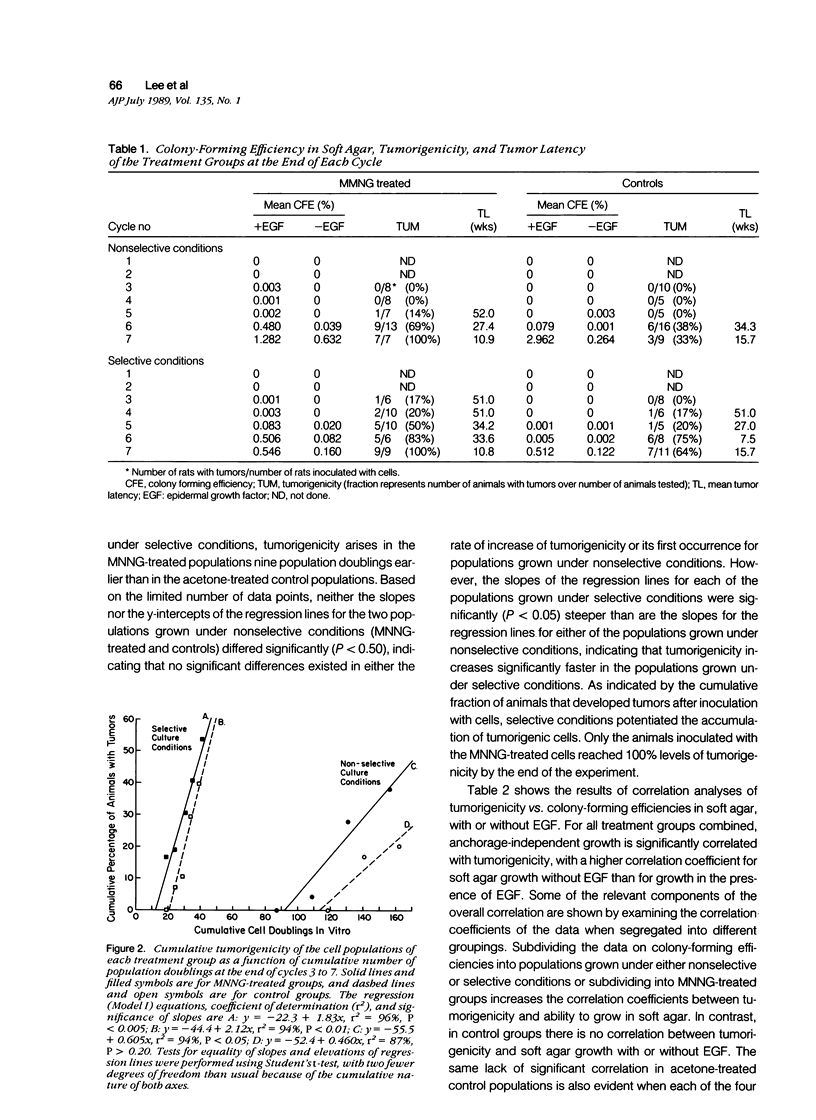
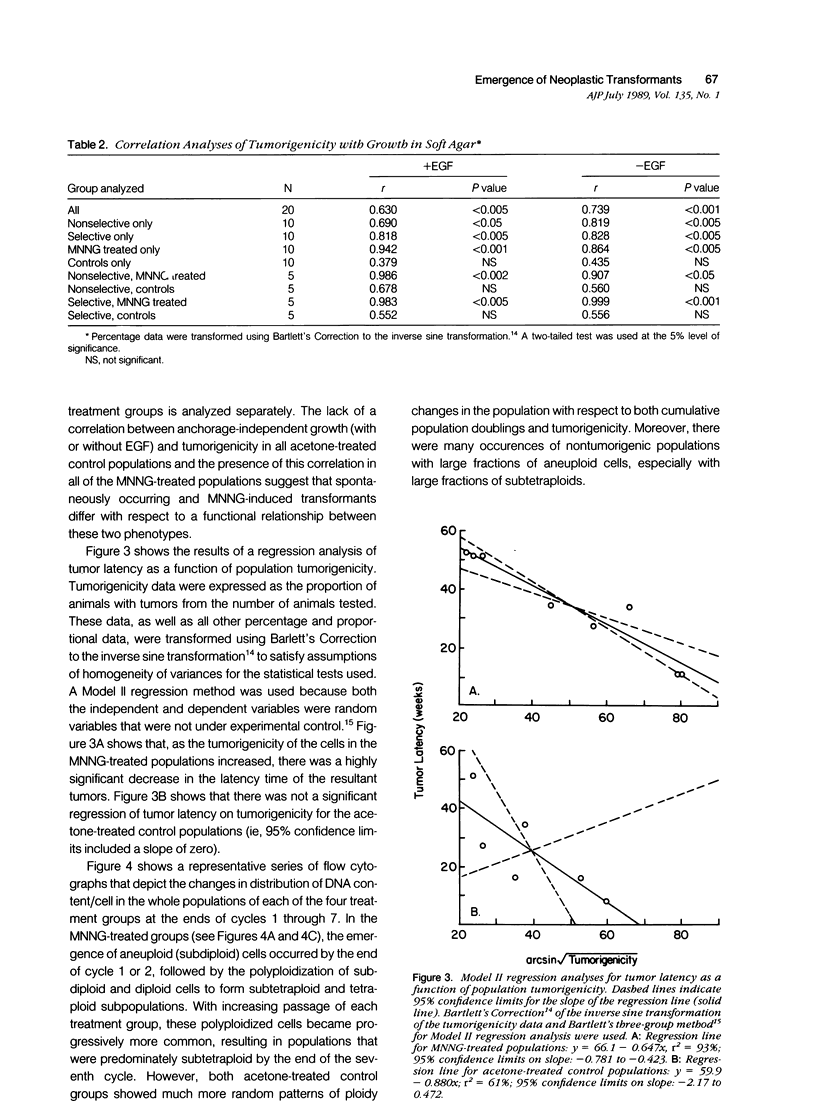
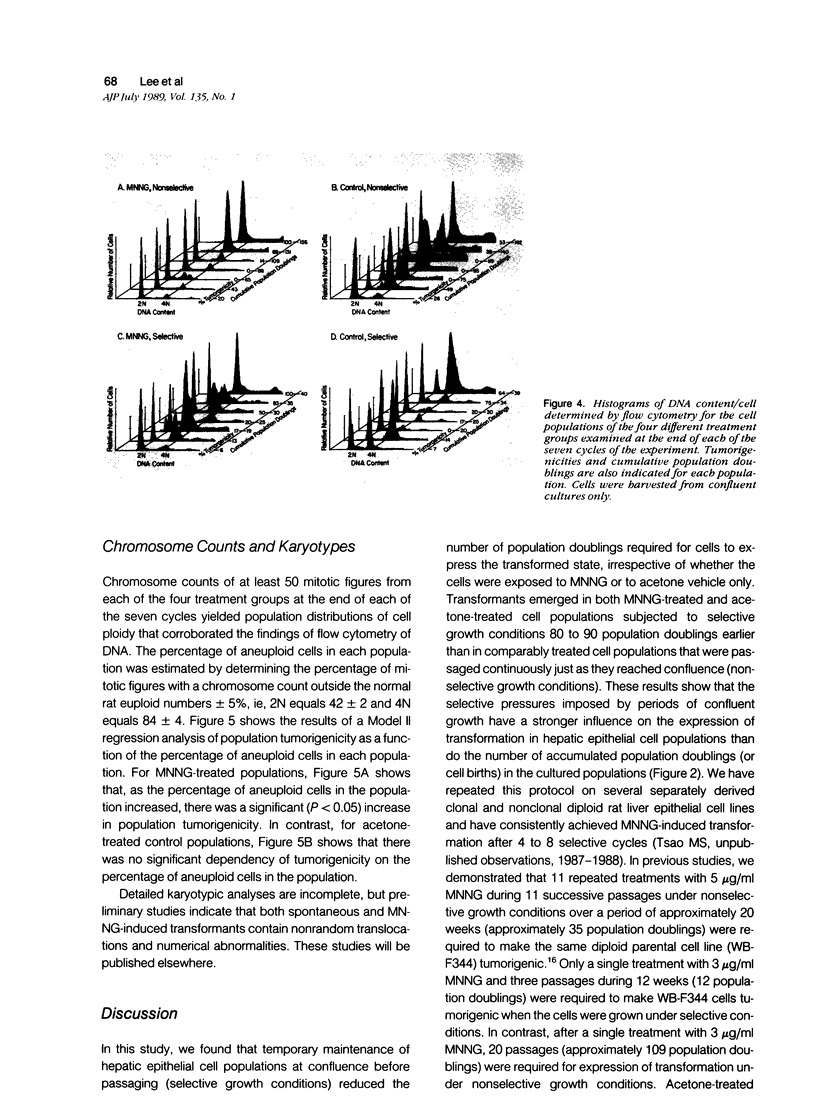
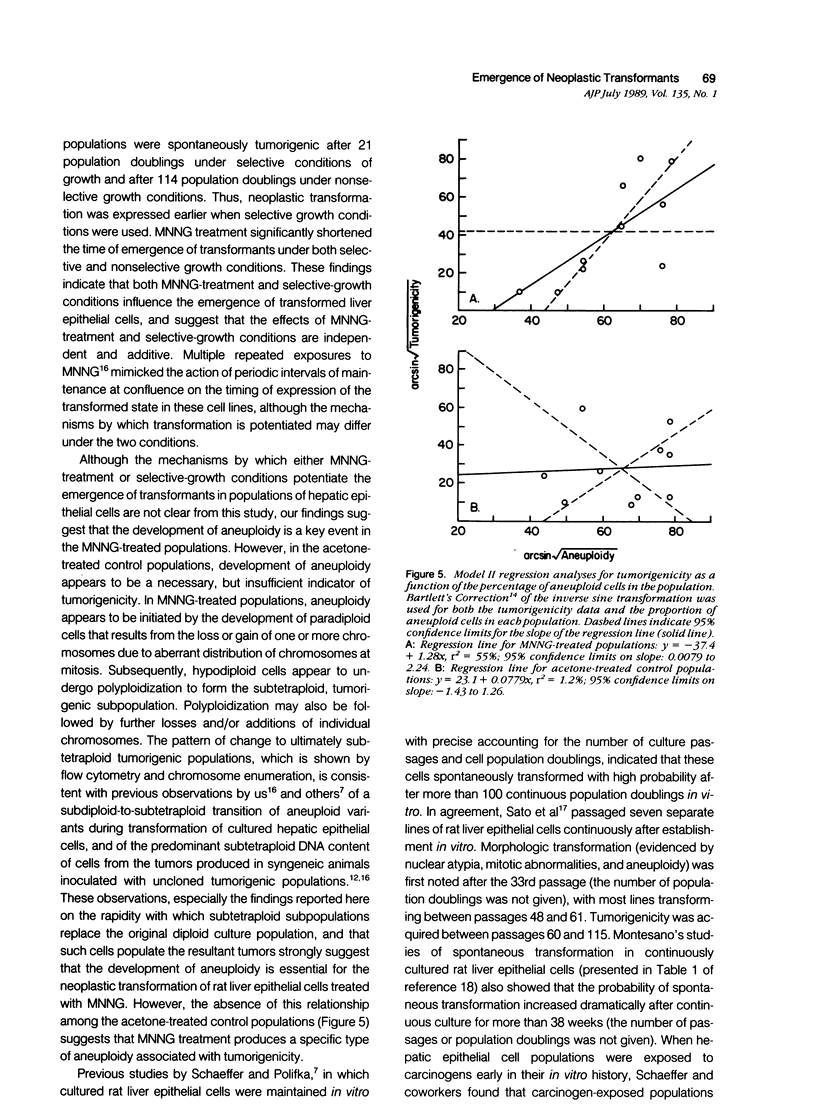
Abstract
Many studies have shown that cultured rat liver epithelial cells can be neoplastically transformed by repeated or long-continued exposure to chemical carcinogens. These cells also may transform spontaneously in the absence of carcinogen treatment after long-term, continuous passage in culture or after chronic maintenance in a confluent state in vitro. In this study, we have compared the times of emergence and rates of accumulation of transformed cells in populations of rat hepatic epithelial cells exposed either to a single dose of N-methyl-N'-nitro-N-nitrosoguanidine (MNNG, 3 micrograms/ml culture medium for 30 minutes) or to acetone vehicle alone (3 microliters/ml culture medium for 30 minutes). Transformation was compared in cell populations that were passaged continuously once a week as they attained a confluent density (nonselective growth conditions), or that were maintained at a confluent density for 3 weeks between passages once a month (selective growth conditions). Emergence of both spontaneous transformants and transformants induced by MNNG was facilitated by selective growth conditions, as compared with non-selective growth conditions. Transformants were detected both in cultures exposed to MNNG and in cultures exposed only to acetone (solvent controls), but they always emerged earlier in cultures exposed to MNNG (nine population doublings earlier when grown under selective growth conditions and 22 population doublings earlier when grown under nonselective growth conditions). Once transformants were detected, they replaced the nontransformed population more quickly under selective than under nonselective conditions of culture. Cells possessing the ability to grow in soft agar and to produce tumors in syngeneic rats were detected (at about 12 population doublings after treatment) under selective conditions much earlier than under nonselective growth conditions (at about 90 population doublings after treatment). Among MNNG-treated cultures, the fraction of aneuploid cells in the population was correlated significantly with tumorigenicity. In contrast, among acetone-treated control populations, aneuploidy and tumorigenicity were not correlated; populations of aneuploid acetone-treated cells often were not tumorigenic. These observations suggest that MNNG treatment produced a specific type of aneuploidy that was associated with tumorigenicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borek C. Neoplastic transformation in vitro of a clone of adult liver epithelial cells into differentiated hepatoma-like cells under conditions of nutritional stress. Proc Natl Acad Sci U S A. 1972 Apr;69(4):956–959. doi: 10.1073/pnas.69.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. D., Wilson M. J., Poirier L. A. Neoplastic conversion of rat liver epithelial cells in culture by ethionine and S-adenosylethionine. Carcinogenesis. 1983;4(2):173–177. doi: 10.1093/carcin/4.2.173. [DOI] [PubMed] [Google Scholar]
- DeLuca J. G., Weinstein L., Thilly W. G. Ultraviolet light-induced mutation of diploid human lymphoblasts. Mutat Res. 1983 Feb;107(2):347–370. doi: 10.1016/0027-5107(83)90176-8. [DOI] [PubMed] [Google Scholar]
- Grisham J. W. Use of hepatic cell cultures to detect and evaluate the mechanisms of action of toxic chemicals. Int Rev Exp Pathol. 1979;20:123–210. [PubMed] [Google Scholar]
- Heintz N., Little B., Bresnick E., Schaeffer W. I. Malignant transformation of cultured rat hepatocytes by (+)- and (-)-trans-7,8-dihydro-7,8-dihydroxybenzo(a)pyrene. Cancer Res. 1980 Apr;40(4):1281–1285. [PubMed] [Google Scholar]
- Montesano R., Drevon C., Kuroki T., Saint Vincent L., Handleman S., Sanford K. K., DeFeo D., Weinstein I. B. Test for malignant transformation of rat liver cells in culture: cytology, growth in soft agar, and production of plasminogen activator. J Natl Cancer Inst. 1977 Dec;59(6):1651–1658. doi: 10.1093/jnci/59.6.1651. [DOI] [PubMed] [Google Scholar]
- Sato J., Namba M., Usui K., Nagano D. Carcinogenesis in tissue culture. 8. Spontaneous malignant transformation of rat liver cells in long-term culture. Jpn J Exp Med. 1968 Apr;38(2):105–118. [PubMed] [Google Scholar]
- Schaeffer W. I., Heintz N. H. A diploid rat liver cell culture. IV. Malignant transformation by aflatoxin B1. In Vitro. 1978 May;14(5):418–427. doi: 10.1007/BF02616103. [DOI] [PubMed] [Google Scholar]
- Schaeffer W. I., Polifka M. D. A diploid rat liver cell culture. III. Characterization of the heteroploid morphological variants which develop with time in culture. Exp Cell Res. 1975 Oct 1;95(1):167–175. doi: 10.1016/0014-4827(75)90622-9. [DOI] [PubMed] [Google Scholar]
- Tokiwa T., Sato J. Effect of 3'-methyl-4-dimethylaminoazobenzene in the induction of malignant transformation and of 8-azaguanine-resistant mutations and chromosomal aberrations in a diploid clone derived from normal rat liver cells in culture. In Vitro. 1982 Jun;18(6):501–509. doi: 10.1007/BF02810072. [DOI] [PubMed] [Google Scholar]
- Tsao M. S., Earp H. S., Grisham J. W. Gradation of carcinogen-induced capacity for anchorage-independent growth in cultured rat liver epithelial cells. Cancer Res. 1985 Sep;45(9):4428–4432. [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W., Chou B. B., Smith J. D. Clonal isolation of populations of gamma-glutamyl transpeptidase-positive and -negative cells from rat liver epithelial cells chemically transformed in vitro. Cancer Res. 1985 Oct;45(10):5134–5138. [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W., Nelson K. G. Clonal analysis of tumorigenicity and paratumorigenic phenotypes in rat liver epithelial cells chemically transformed in vitro. Cancer Res. 1985 Oct;45(10):5139–5144. [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W., Nelson K. G., Smith J. D. Phenotypic and karyotypic changes induced in cultured rat hepatic epithelial cells that express the "oval" cell phenotype by exposure to N-methyl-N'-nitro-N-nitrosoguanidine. Am J Pathol. 1985 Feb;118(2):306–315. [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W. Phenotypic modulation during tumorigenesis by clones of transformed rat liver epithelial cells. Cancer Res. 1987 Mar 1;47(5):1282–1286. [PubMed] [Google Scholar]
- Tsao M. S., Smith J. D., Nelson K. G., Grisham J. W. A diploid epithelial cell line from normal adult rat liver with phenotypic properties of 'oval' cells. Exp Cell Res. 1984 Sep;154(1):38–52. doi: 10.1016/0014-4827(84)90666-9. [DOI] [PubMed] [Google Scholar]



